Abstract
Purpose
To evaluate the PK and safety of siponimod, a substrate of CYP2C9/3A4, in the presence or absence of a CYP3A4 inhibitor, itraconazole.
Methods
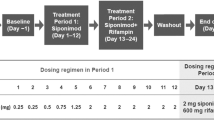
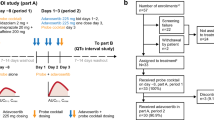
This was an open-label study in healthy subjects (aged 18–50 years; genotype: CYP2C9 *1*2 [cohort 1; n = 17] or *1*3 [cohort 2; n = 13]). Subjects received siponimod 0.25-mg single dose in treatment period 1 (days 1–14), itraconazole 100 mg twice daily in treatment period 2 (days 15–18), and siponimod 0.25-mg single dose (day 19) with itraconazole until day 31 (cohort 1) or day 35 (cohort 2) in treatment period 3. PK of siponimod alone and with itraconazole and safety were assessed.
Results
Overall, 29/30 subjects completed the study. In treatment period 1, geometric mean AUCinf, T1/2, and median Tmax were higher while systemic clearance was lower in cohort 2 than cohort 1. In treatment period 3, siponimod AUC decreased by 10% (geo-mean ratio [90% confidence intervals]: 0.90 [0.84; 0.96]) and 24% (0.76 [0.69; 0.82]) in cohorts 1 and 2, respectively. Siponimod Cmax was similar between treatment periods 1 and 3. In both cohorts, the Cmax and AUC of the metabolites (M17, M3, and M5) decreased in the presence of itraconazole. All adverse events were mild.
Conclusions
The minor albeit significant reduction in plasma exposure of siponimod and its metabolites by itraconazole was unexpected. While the reason is unclear, the results suggest that coadministration of the two drugs would not cause a considerable increase of siponimod exposure independent of CYP2C9 genotype.



Similar content being viewed by others
References
Koch MW, Cutter G, Stys PK, Yong VW, Metz LM (2013) Treatment trials in progressive MS--current challenges and future directions. Nat Rev Neurol 9(9):496–503. https://doi.org/10.1038/nrneurol.2013.148
Atlas of MS 2013. http://www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS.pdf (accessed on 20 June, 2019)
Lublin FD, Reingold SC (1996) Defining the clinical course of multiple sclerosis. Results of an international survey 46 (4): 907–911 DOI https://doi.org/10.1212/wnl.46.4.907
Scalfari A, Neuhaus A, Degenhardt A, Rice GP, Muraro PA, Daumer M, Ebers GC (2010) The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain 133 (Pt 7): 1914–1929 DOI https://doi.org/10.1093/brain/awq118
Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, Sormani MP, Thalheim C, Traboulsee A, Vollmer T (2016) Brain health: time matters in multiple sclerosis. Mult Scler Relat Dis 9:S5–S48. https://doi.org/10.1016/j.msard.2016.07.003
Brinkmann V (2007) Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 115(1):84–105. https://doi.org/10.1016/j.pharmthera.2007.04.006
Gergely P, Nuesslein-Hildesheim B, Guerini D, Brinkmann V, Traebert M, Bruns C, Pan S, Gray NS, Hinterding K, Cooke NG, Groenewegen A, Vitaliti A, Sing T, Luttringer O, Yang J, Gardin A, Wang N, Crumb WJ Jr, Saltzman M, Rosenberg M, Wallstrom E (2012) The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol 167(5):1035–1047. https://doi.org/10.1111/j.1476-5381.2012.02061.x
Mannioui A, Vauzanges Q, Fini JB, Henriet E, Sekizar S, Azoyan L, Thomas JL, Pasquier DD, Giovannangeli C, Demeneix B, Lubetzki C, Zalc B (2017) The Xenopus tadpole: an in vivo model to screen drugs favoring remyelination. Mult Scler Houndmills, Basingstoke, England 24:1352458517721355. https://doi.org/10.1177/1352458517721355
Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, Walsh FS, Pangalos MN, Arimura N, Kaibuchi K, Zalc B, Lubetzki C (2005) Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci 25(6):1459–1469. https://doi.org/10.1523/jneurosci.4645-04.2005
Kappos L, Bar-Or A, Cree BAC, Fox RJ, Giovannoni G, Gold R, Vermersch P, Arnold DL, Arnould S, Scherz T, Wolf C, Wallström E, Dahlke F (2018) Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 391(10127):1263–1273. https://doi.org/10.1016/S0140-6736(18)30475-6
Prescribing information: Mayzent (siponimod). East Hanover, NJ: Novartis pharmaceuticals, 2019 (package insert)
Glaenzel U, Jin Y, Nufer R, Li W, Schroer K, Adam-Stitah S, Peter van Marle S, Legangneux E, Borell H, James AD, Meissner A, Camenisch G, Gardin A (2018) Metabolism and disposition of Siponimod, a novel selective S1P1/S1P5 agonist, in healthy volunteers and in vitro identification of human cytochrome P450 enzymes involved in its oxidative metabolism. Drug Metab Dispos 46(7):1001–1013. https://doi.org/10.1124/dmd.117.079574
Jin Y, Borell H, Gardin A, Ufer M, Huth F, Camenisch G (2018) In vitro studies and in silico predictions of fluconazole and CYP2C9 genetic polymorphism impact on siponimod metabolism and pharmacokinetics. Eur J Clin Pharmacol 74(4):455–464. https://doi.org/10.1007/s00228-017-2404-2
Gardin A, Ufer M, Legangneux E, Rossato G, Jin Y, Su Z, Pal P, Li W, Shakeri-Nejad K (2019) Effect of fluconazole Coadministration and CYP2C9 genetic polymorphism on Siponimod pharmacokinetics in healthy subjects. Clin Pharmacokinet 58(3):349–361. https://doi.org/10.1007/s40262-018-0700-3
Pharmacogene Variation Consortium (PharmVar). https://www.pharmvar.org/gene/CYP2C9. Accessed 1 April 2019
Hirota T, Eguchi S, Ieiri I (2013) Impact of genetic polymorphisms in CYP2C9 and CYP2C19 on the pharmacokinetics of clinically used drugs. Drug Metab Pharmacokinetics 28(1):28–37
Van Booven D, Marsh S, McLeod H, Carrillo MW, Sangkuhl K, Klein TE, Altman RB (2010) Cytochrome P450 2C9-CYP2C9. Pharmacogenet Genomics 20(4):277–281. https://doi.org/10.1097/FPC.0b013e3283349e84
Huth F, Gardin A, Umehara KI, He H (2019) Prediction of the impact of CYP2C9 genotypes on the drug-drug interaction potential of Siponimod with PBPK modeling: a comprehensive approach for drug label recommendations. Clin Pharmacol Ther. https://doi.org/10.1002/cpt.1547
Isoherranen N, Kunze KL, Allen KE, Nelson WL, Thummel KE (2004) Role of itraconazole metabolites in CYP3A4 inhibition. Drug Metab Dispos 32(10):1121–1131. https://doi.org/10.1124/dmd.104.000315
Krasulova K, Dvorak Z, Anzenbacher P (2019) In vitro analysis of itraconazole cis-diastereoisomers inhibition of nine cytochrome P450 enzymes: stereoselective inhibition of CYP3A. Xenobiotica 49(1):36–42. https://doi.org/10.1080/00498254.2018.1425510
Guidance for Industry Drug interaction studies—study design, data analysis, implications for dosing, and labeling recommendation. Draft guidance (2017) https://www.fda.gov/downloads/drugs/guidances/ucm292362.pdf (Accessed on 20 June, 2019)
Negroni R, Arechavala AI (1993) Itraconazole: pharmacokinetics and indications. Arch Med Res 24(4):387–393
Templeton I, Peng C-C, Thummel K, Davis C, Kunze K, Isoherranen N (2010) Accurate prediction of dose-dependent CYP3A4 inhibition by Itraconazole and its metabolites from in vitro inhibition data. Clin Pharmacol Ther 88(4):499–505. https://doi.org/10.1038/clpt.2010.119
Kirchheiner J, Brockmöller J (2005) Clinical consequences of cytochrome P450 2C9 polymorphisms. Clin Pharmacol Ther 77(1):1–16. https://doi.org/10.1016/j.clpt.2004.08.009
Scott SA, Khasawneh R, Peter I, Kornreich R, Desnick RJ (2010) Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics 11(6):781–791. https://doi.org/10.2217/pgs.10.49
Hynninen VV, Olkkola KT, Bertilsson L, Kurkinen KJ, Korhonen T, Neuvonen PJ, Laine K (2009) Voriconazole increases while Itraconazole decreases plasma meloxicam concentrations. Antimicrob Agents Chemother 53(2):587–592. https://doi.org/10.1128/aac.00530-08
Kim JH, Sherman ME, Curriero FC, Guengerich FP, Strickland PT, Sutter TR (2004) Expression of cytochromes P450 1A1 and 1B1 in human lung from smokers, non-smokers, and ex-smokers. Toxicol Appl Pharmacol 199(3):210–219. https://doi.org/10.1016/j.taap.2003.11.015
Korashy HM, Shayeganpour A, Brocks DR, El-Kadi AO (2007) Induction of cytochrome P450 1A1 by ketoconazole and itraconazole but not fluconazole in murine and human hepatoma cell lines. Toxicol Sci:Off J Soc Toxicol 97(1):32–43. https://doi.org/10.1093/toxsci/kfm012
Gardin A, Gray C, Neelakantham S, Huth F, Davidson AM, Dumitras S, Legangneux E, Shakeri-Nejad K (2018) Siponimod pharmacokinetics, safety, and tolerability in combination with rifampin, a CYP2C9/3A4 inducer, in healthy subjects. Eur J Clin Pharmacol 74:1593–1604. https://doi.org/10.1007/s00228-018-2533-2
World Medical Association. WMA Declaration of Helsinki—ethical principles for medical research involving human subjects. https://www.wma.net/wp-content/uploads/2018/07/DoH-Oct2008.pdf (accessed on 20 June, 2019)
Acknowledgments
The authors would like to acknowledge Stuart Harris (the principal investigator) who contributed to the protocol development and data analysis, Vikram Kanmathareddy who was the trial programmer and Subhajit Choudhury for his assistance/contribution to the statistical design and analysis. They would also like to acknowledge Arshjyoti Singh of Novartis Healthcare Pvt. Ltd. Hyderabad, India, for providing medical writing support, which encompassed preparing the manuscript, formatting, referencing, preparing tables and figures, incorporating authors’ revisions, finalizing and submission, all under the direction of the authors and Sivaram Vedantam (Novartis Healthcare Pvt. Ltd.) for review support. All authors edited the manuscript for intellectual content, provided guidance during manuscript development, and approved the final version submitted for publication.
Funding
This study was funded by Novartis Pharma AG, Switzerland.
Author information
Authors and Affiliations
Contributions
Anne Gardin (pharmacokinetics) and Kasra Shakeri-Nejad (medical, safety) conceptualized and designed the study, contributed to the protocol development and study conduct, analyzed and interpreted the study data, and contributed to the study report preparation. Andrea Feller contributed to the protocol development, study conduct, and study report preparation. Felix Huth contributed to the conceptualization and interpretation of the data. Srikanth Neelakantham was the trial statistician. Swati Dumitras supported the conceptualization of the study, provided critical revision of the manuscript for the content, and supervised the overall research. All authors made a significant contribution to data interpretation and review of the manuscript.
Corresponding author
Ethics declarations
The study was conducted at two centers namely, SeaView Research, Inc., Miami, Florida, USA and SeaView Jacksonville, LLC Jacksonville, Florida, USA. The study protocol and subject consent forms were reviewed and approved by the Schulman Institutional Review Board, Cincinnati OH, USA. The study was conducted according to the ethical principles of the Declaration of Helsinki [30] and designed in compliance with the FDA guidelines for industry on Drug Interaction Studies-Study Design, Data Analysis, Implications for Dosing and Labeling Recommendation [21].
Conflict of interest
Anne Gardin, Andrea Feller, Felix Huth, Srikanth Neelakantham, Swati Dumitras, and Kasra Shakeri-Nejad are employees of Novartis.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 387 kb)
Rights and permissions
About this article
Cite this article
Gardin, A., Shakeri-Nejad, K., Feller, A. et al. Siponimod pharmacokinetics, safety, and tolerability in combination with the potent CYP3A4 inhibitor itraconazole in healthy subjects with different CYP2C9 genotypes. Eur J Clin Pharmacol 75, 1565–1574 (2019). https://doi.org/10.1007/s00228-019-02729-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02729-7