Abstract
Aim
The aim of the present study was to investigate the existence of time-dependent pharmacokinetics of artesunate (ARS) during 5 consecutive days of oral administration to 10 healthy Vietnamese subjects (aged 21–52 years and weighing 49–90 kg).
Methods
Each volunteer received 200 mg oral doses of ARS once daily for 5 consecutive days. Blood samples (3 ml each) were collected on days 1 and 5 at 0 (before dosing), 0.5, 1, 2, 3, 4, and 6 h after drug administration. During days 2, 3, and 4, the same volumes of blood were collected at 0, 1, 2, and 4 h after dosing. Plasma ARS and dihydroartemisinin (DHA) were measured using high-performance liquid chromatography–mass spectrometry (LC-MS).
Results
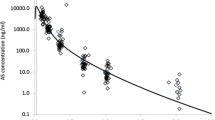
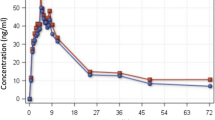
Results did not show evidence of time-dependency for ARS or the active plasma metabolite DHA. There were no differences in the concentrations of ARS and DHA at all sampling times on days 1 and 5. In addition, the pharmacokinetics of both compounds were similar on days 1 and 5. This finding confirms that the enzyme auto-induction in drug metabolism may not be characteristic of the endoperoxide sesquiterpene antimalarial group.


Similar content being viewed by others
References
Li GQ, Guo XB, Fu LC, Jian HX, Wang XH (1994) Clinical trials of artemisinin and its derivatives in the treatment of malaria in China. Trans R Soc Trop Med Hyg 88(Suppl 1):S5–S6
Ashton M, Hai TN, Sy ND, Huong DX, Van Huong N, Niêu NT, Công LD (1998) Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab Dispos 26:25–27
Ashton M, Gordi T, Trinh NH, Nguyen VH, Nguyen DS, Nguyen TN, Dinh XH, Johansson M, Le DC (1998) Artemisinin pharmacokinetics in healthy adults after 250, 500 and 1000 mg single oral doses. Biopharm Drug Dispos 19:245–250
Ashton M, Nguyen DS, Nguyen VH, Gordi T, Trinh NH, Dinh XH, Nguyen TN, Le DC (1998) Artemisinin kinetics and dynamics during oral and rectal treatment of uncomplicated malaria. Clin Pharmacol Ther 63:482–493
Alin MH, Ashton M, Kihamia CM, Mtey GJ, Björkman A (1996) Clinical efficacy and pharmacokinetics of artemisinin monotherapy and in combination with mefloquine in patients with falciparum malaria. Br J Clin Pharmacol 4:587–592
Hassan Alin M, Ashton M, Kihamia CM, Mtey GJ, Björkman A (1996) Multiple dose pharmacokinetics of oral artemisinin and comparison of its efficacy with that of oral artesunate in falciparum malaria patients. Trans R Soc Trop Med Hyg 90:61–65
Khanh NX, de Vries PJ, Ha LD, van Boxtel CJ, Koopmans R, Kager PA (1999) Declining concentrations of dihydroartemisinin in plasma during 5-day oral treatment with artesunate for falciparum malaria. Antimicrob Agents Chemother 43:690–692
Elsherbiny DA, Asimus SA, Karlsson MO, Ashton M, Simonsson US (2008) A model based assessment of the CYP2B6 and CYP2C19 inductive properties by artemisinin antimalarials: implications for combination regimens. J Pharmacokinet Pharmacodyn 10928:9084–9096
Diem Thuy LT, Ngoc Hung L, Danh PT, Na-Bangchang K (2008) Pharmacokinetics of a five-day oral dihydroartemisinin monotherapy regimen in patients with uncomplicated falciparum malaria. Drug Met Pharmacokinet (in press)
Diem Thuy LT, Ngoc Hung L, Danh PT, Na-Bangchang K (2008) Development and validation of a liquid chromatography-mass spectrometry method for the simultaneous quantification of artesunate and dihydroartemisininin human plasma (submitted)
d’ Argenio DZ, Schumitzky A (2003) ADAPT II, release 4: pharmacokinetic/pharmacodynamic system analysis software. http://bmsr.usc.edu/Software/ADAPT/ADAPT.html. Accessed 14 May 2008
Na-Bangchang K, Krudsood S, Silachamroon U, Molunto P, Tasanor O, Chalermrut K, Tangpukdee O, Kano S, Looareesuwan S (2004) The pharmacokinetics of oral dihydroartemisinin and artesunate in healthy Thai volunteers. Southeast Asian J Trop Med Pub Health 35:575–580
Batty KT, Ashton M, Ilett KF, Edwards G, Davis TM (1998) The pharmacokinetics of artemisinin (ART) and artesunate (ARTS) in healthy volunteers. Am J Trop Med Hyg 58:125–126
Molyneux ME, Looareesuwan S, Menzies IS, Grainger SL, Phillips RE, Wattanagoon Y, Thompson RP, Warrell DA (1989) Reduced hepatic blood flow and intestinal malabsorption in severe falciparum malaria. Am J Trop Med Hyg 40:470–476
Ilett KF, Ethell BT, Maggs JL, Davis TM, Batty KT, Burchell B, Binh TQ, Thu le TA, Hung NC, Pirmohamed M, Park BK, Edwards G (2002) Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP- glucuronosyltransferases. Drug Metab Dispos 30:1005–1012
Svensson US, Ashton M, Trinh NH, Bertilsson L, Dinh XH, Nguyen VH, Nguyen TN, Nguyen DS, Lykkesfeldt J, Le DC (1998) Artemisinin induces omeprazole metabolism in human beings. Clin Pharmacol Ther 64:160–167
Svensson US, Ashton M (1999) Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br J Clin Pharmacol 48:528–535
Olliaro PL, Nair NK, Sathasivam K, Mansor SM, Navaratnam V (2002) Pharmacokinetics of artesunate after single oral administration to rats. BMC Pharmacol 1:12–15
Grace JM, Aguilar AJ, Trotman KM, Peggins JO, Brewer TG (1998) Metabolism of beta-arteether to dihydroqinghaosu by human liver microsomes and recombinant cytochrome P450. Drug Metab Dispos 26:313–317
Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP (1994) Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 270:414–423
Mutabingwa TK (2005) Artemisinin-based combination therapies (ACTs): best hope for malaria treatment but inaccessible to the needy! Acta Trop 95:305–315
Acknowledgements
The study was supported by Thammasat University and UNDP-UNICEF Special Program in Research and Training in Tropical Diseases. We thank Dr. Matthew J. Cheesman for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diem Thuy, L.T., Ngoc Hung, L., Danh, P.T. et al. Absence of time-dependent artesunate pharmacokinetics in healthy subjects during 5-day oral administration. Eur J Clin Pharmacol 64, 993–998 (2008). https://doi.org/10.1007/s00228-008-0506-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0506-6