Abstract
Purpose
To determine the effects of steady-state concentrations of the selective S1P1 receptor modulator ponesimod on the pharmacokinetics (PK) of a single dose of a combined oral contraceptive, containing 1 mg norethisterone (NET) and 35 μg ethinyl estradiol (EE) and to investigate the effects on heart rate at different ponesimod doses within an up-titration regimen prior to co-administration of the contraceptive.
Methods
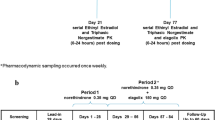
Twenty-two healthy women (age: 29-60 years) received twice a single oral dose of the combined oral contraceptive, alone or in combination with multiple doses of 40 mg ponesimod attained by an up-titration regimen. Heart rate (HR) effects were assessed on the first day of each up-titration level. PK parameters of NET and EE were determined by non-compartmental analysis.
Results
Geometric mean ratios (ponesimod and contraceptive / contraceptive alone) of Cmax and AUC0-24 of NET were 0.87 (90 % CI: 0.80, 0.94) and 0.84 (90 % CI: 0.76, 0.93), respectively. Geometric mean ratios of Cmax and AUC0-24 of EE were 0.94 (90 % CI: 0.86, 1.03) and 0.95 (90 % CI: 0.89, 1.01), respectively. The maximum mean HR reduction after the first dose of 10 mg ponesimod was 12.4 bpm (SD ± 6.2) at 2.5 h post-dose. On Day 4 (first dose of 20 mg) and Day 7 (first dose of 40 mg) the maximum mean HR reduction was 4.3 bpm (SD ± 5.7) and 1.4 (SD ± 6.4), respectively, at 2.5 h post-dose compared to baseline.
Conclusion
No clinically relevant PK interactions between ponesimod and the combined oral contraceptive were observed, therefore, efficacy of hormonal contraceptives is not expected to be affected by concomitant administration of ponesimod. The up-titration regimen showed that HR reductions are diminished upon repeated ponesimod administration.




Similar content being viewed by others
Abbreviations
- NET:
-
Norethisterone
- EE:
-
Ethinyl estradiol
References
Bolli MH, Abele S, Binkert C, Bravo R, Buchmann S, Bur D, Gatfield J, Hess P, Kohl C, Mangold C, Mathys B, Menyhart K, Muller C, Nayler O, Scherz M, Schmidt G, Sippel V, Steiner B, Strasser D, Treiber A, Weller T (2010) 2-imino-thiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J Med Chem 53(10):4198–4211
Piali L, Froidevaux S, Hess P, Nayler O, Bolli MH, Schlosser E, Kohl C, Steiner B, Clozel M (2011) The selective sphingosine 1-phosphate receptor 1 agonist ponesimod protects against lymphocyte-mediated tissue inflammation. J Pharmacol Exp Ther 337(2):547–556
Brossard P, Derendorf H, Xu J, Maatouk H, Halabi A, Dingemanse J (2013) Pharmacokinetics and Pharmacodynamics of Ponesimod, a Selective S1P Receptor Modulator, in the First-in-Human Study. Br J Clin Pharmacol 76(6):888–896
Braley TJ, Segal BM (2013) B-Cell Targeting Agents in the Treatment of Multiple Sclerosis. Curr Treat Options Neurol. doi:10.1007/s11940-013-0232-y
Cai Y, Fleming C, Yan J (2012) New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol 9(4):302–309
Zaguia F, Saikali P, Ludwin S, Newcombe J, Beauseigle D, McCrea E, Duquette P, Prat A, Antel JP, Arbour N (2013) Cytotoxic NKG2C + CD4 T cells target oligodendrocytes in multiple sclerosis. J Immunol 190(6):2510–2518
Gambichler T, Zhang Y, Hoxtermann S, Kreuter A (2013) Natural killer cells and B lymphocytes in peripheral blood of patients with psoriasis. Br J Dermatol 168(4):894–896
Olsson T, Boster A, Fernández Ó, Freedman MS, Pozzilli C, Bach D, Berkani O, Mueller MS, Sidorenko T, Melanson M (2012) Efficacy and safety of ponesimod, an oral, selective sphingosine 1-phosphate receptor-1 modulator, in patients with relapsing-remitting multiple sclerosis: Results from a phase IIb, randomised, double-blind, placebo-controlled trial. Multiple Sclerosis Journal 18 (4 suppl) (49): 152
Freedman MS, Olsson T, Melanson M, Fernández Ó, Boster A, Bach D, Berkani O, Mueller MS, Sidorenko T, Pozzilli C (2012) Dose-dependent effect of ponesimod, an oral, selective sphingosine 1-phosphate receptor-1 modulator, on magnetic resonance imaging outcomes in patients with relapsing-remitting multiple sclerosis. Multiple Sclerosis Journal 18 (4 Suppl) (420): 923
Fernández Ó, Pozzilli C, Freedman MS, Olsson T, Melanson M, Bach D, Berkani O, Mueller MS, Sidorenko T, Boster A (2012) Pharmacodynamic effect, safety and tolerability of ponesimod, a selective sphingosine 1-phosphate receptor-1 modulator, in patients with relapsing-remitting multiple sclerosis. Multiple Sclerosis Journal 18 (4 Suppl):417
Chimenti S, Arenberger P, Karpati S, Vaclavkova A, Burcklen M, Stefani M, D’Ambrosio D (2013) A phase II study of ponesimod, an oral, selective sphingosine-1-phosphate receptor-1 modulator in chronic plaque psoriasis. 4th Congress of the Psoriasis Network, Paris, France
Brossard P, Maatouk H, Halabi A, Dingemanse J (2012) Ascending multiple-dose study with ponesimod, a selective S1P1 receptor agonist: tolerability, safety, pharmacokinetics, and pharmacodynamics. Clin Pharmacol Ther 91:S93
Tseng A, Hills-Nieminen C (2013) Drug interactions between antiretrovirals and hormonal contraceptives. Expert Opin Drug Metab Toxicol 9(5):559–572
Barditch-Crovo P, Trapnell CB, Ette E, Zacur HA, Coresh J, Rocco LE, Hendrix CW, Flexner C (1999) The effects of rifampin and rifabutin on the pharmacokinetics and pharmacodynamics of a combination oral contraceptive. Clin Pharmacol Ther 65(4):428–438
Dutton C, Foldvary-Schaefer N (2008) Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol 83:113–134
Hall SD, Wang Z, Huang SM, Hamman MA, Vasavada N, Adigun AQ, Hilligoss JK, Miller M, Gorski JC (2003) The interaction between St John’s wort and an oral contraceptive. Clin Pharmacol Ther 74(6):525–535
Zhang H, Cui D, Wang B, Han YH, Balimane P, Yang Z, Sinz M, Rodrigues AD (2007) Pharmacokinetic drug interactions involving 17alpha-ethinylestradiol: a new look at an old drug. Clin Pharmacokinet 46(2):133–157
Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J (2005) General considerations for lung function testing. Eur Respir J 26(1):153–161
Schwartz JB (1999) Oral contraceptive therapy in women: drug interactions and unwanted outcomes. J Gend Specif Med 2(6):26–29
LeBel M, Masson E, Guilbert E, Colborn D, Paquet F, Allard S, Vallee F, Narang PK (1998) Effects of rifabutin and rifampicin on the pharmacokinetics of ethinylestradiol and norethindrone. J Clin Pharmacol 38(11):1042–1050
WHO (2009) Department of Reproductive Health WHO. Medical eligibility criteria for contraceptive use. World Health Organization Fourth edition ed
Sekar VJ, Lefebvre E, Guzman SS, Felicione E, De Pauw M, Vangeneugden T, Hoetelmans RM (2008) Pharmacokinetic interaction between ethinyl estradiol, norethindrone and darunavir with low-dose ritonavir in healthy women. Antivir Ther 13(4):563–569
Wang B, Sanchez RI, Franklin RB, Evans DC, Huskey SE (2004) The involvement of CYP3A4 and CYP2C9 in the metabolism of 17 alpha-ethinylestradiol. Drug Metab Dispos 32(11):1209–1212
Hilbert J, Messig M, Kuye O, Friedman H (2001) Evaluation of interaction between fluconazole and an oral contraceptive in healthy women. Obstet Gynecol 98(2):218–223
Rosenfeld WE, Doose DR, Walker SA, Nayak RK (1997) Effect of topiramate on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in patients with epilepsy. Epilepsia 38(3):317–323
Sinofsky FE, Pasquale SA (1998) The effect of fluconazole on circulating ethinyl estradiol levels in women taking oral contraceptives. Am J Obstet Gynecol 178(2):300–304
Tsuchiya Y, Nakajima M, Yokoi T (2005) Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett 227(2):115–124
Ouellet D, Hsu A, Qian J, Locke CS, Eason CJ, Cavanaugh JH, Leonard JM, Granneman GR (1998) Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. Br J Clin Pharmacol 46(2):111–116
Back DJ, Orme ML (1990) Pharmacokinetic drug interactions with oral contraceptives. Clin Pharmacokinet 18(6):472–484
Rivera R, Yacobson I, Grimes D (1999) The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol 181(5 Pt 1):1263–1269
Lee R (2009) Drug interactions and hormonal contraception. Trend Urol Gyneacol Sex Health 14(3):23–26
Acknowledgments
The authors would like to thank Dr Dikran Mouradian, principal investigator of this study, Marzia Cavallaro for the statistical analysis, Kasra Shakeri-Nejad and Wenting Zhang-Fu for their medical input and assessment, Swiss Bioanalytics, Birsfelden, Switzerland for analysis of ponesimod, and PPD Richmond, VA, USA for analysis of NET and EE.
Conflict of interest/Disclosure
Actelion Pharmaceuticals Ltd provided the funding for this clinical trial. Maribel Reyes, Matthias Hoch, and Jasper Dingemanse are full time employees of Actelion Pharmaceuticals Ltd. Patrick Brossard is a former employee of Actelion Pharmaceuticals Ltd. Didier Chassard is a full time employee of Biotrial.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
Maribel Reyes was the lead author, and wrote, and directed the manuscript content of each draft. Patrick Brossard and Jasper Dingemanse designed and evaluated the research. Didier Chassard and Matthias Hoch carefully reviewed the manuscript drafts. All authors reviewed, interpreted the data, and agreed on the content. All authors approved the final version for submission.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 46.9 kb)
Rights and permissions
About this article
Cite this article
Reyes, M., Brossard, P., Chassard, D. et al. Effects of ponesimod, a selective S1P1 receptor modulator, on the pharmacokinetics of a hormonal combination contraceptive. Eur J Clin Pharmacol 70, 287–293 (2014). https://doi.org/10.1007/s00228-013-1625-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1625-2