Abstract
Purpose
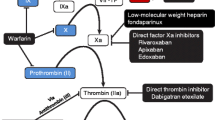
Vorapaxar is an orally active protease-activated receptor 1 (PAR-1) antagonist that inhibits thrombin-induced platelet aggregation. This open-label study assessed the pharmacokinetics and pharmacodynamics of single-dose warfarin in the presence/absence of multiple-dose vorapaxar in 12 healthy men.
Methods
Subjects received two treatments separated by ≥7-day washout: Treatment A warfarin 25 mg (Day 1); Treatment B vorapaxar 2.5 mg/day on Days 1–6 and vorapaxar 40 mg coadministered with warfarin 25 mg (Day 7). R-warfarin, S-warfarin, and prothrombin time (PT) were assayed predose and up to 120 h postdose.
Results
The geometric mean ratio (GMR) as a percentage (warfarin + vorapaxar/warfarin) was calculated. The GMR (90 % CIs) estimates of Cmax were 105 (99, 111) and 105 (99, 112) for R- and S-warfarin, respectively. The GMR (90 % CIs) estimates of AUC0-∞ were 108 (101, 116) and 105 (96, 115) for R- and S-warfarin, respectively. The GMR (95 % CIs) estimates of AUC0–120 h for PT and INR were 97 (95, 98) and 96 (94, 98), respectively.
Conclusion
Results of this study indicate that vorapaxar has no meaningful effect on the pharmacokinetics or pharmacodynamics of warfarin, suggesting that the coadministration of these two drugs or vorapaxar coadministered with other CYP2C9/CYP2C19 substrates is unlikely to cause a clinically significant pharmacokinetic drug interaction.


Similar content being viewed by others
References
Chackalamannil S, Wang Y, Greenlee WJ, Hu Z, Xia Y, Ahn HS, Boykow G, Hsieh Y, Palamanda J, Agans-Fantuzzi J et al (2008) Discovery of a novel, orally active himbacine-based thrombin receptor antagonist (SCH 530348) with potent antiplatelet activity. J Med Chem 51:3061–3064
Chintala M, Vemulapalli S, Kurowski S, Sabin C, Reynolds D, Prevete K, Friedrichs G (2008) SCH 530348, a novel oral antiplatelet agent, demonstrated no bleeding risk alone or in combination with aspirin and clopidogrel in cynomolgus monkeys. Atheroscler Thromb Vasc Biol e-136
Kosoglou T, Reyderman L, Tiessen RG, van Vliet A, Fales RR, Keller R, Yang B, Cutler DL (2012) Pharmacodynamics and pharmacokinetics of the novel PAR-1 antagonist vorapaxar (formerly SCH 530348) in healthy subjects. Eur J Clin Pharmacol 68:249–258
Goto S, Yamaguchi T, Ikeda Y, Kato K, Yamaguchi H, Jensen P (2010) Safety and exploratory efficacy of the novel thrombin receptor (PAR-1) antagonist SCH530348 for non-ST-segment elevation acute coronary syndrome. J Atheroscler Thromb 17:156–164
Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E et al (2012) Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med 366:20–33
Morrow DA, Scirica BM, Fox KA, Berman G, Strony J, Veltri E, Bonaca MP, Fish P, McCabe CH, Braunwald E (2009) Evaluation of a novel antiplatelet agent for secondary prevention in patients with a history of atherosclerotic disease: design and rationale for the thrombin-receptor antagonist in secondary prevention of atherothrombotic ischemic events (TRA 2 degrees P)-TIMI 50 trial. Am Heart J 158:335–341
Coumadin Tablets/Coumadin for Injection [prescribing information] (2010) Bristol-Myers Squibb, Princeton, NJ
Hirsh J (1991) Oral anticoagulant drugs. N Engl J Med 324:1865–1875
Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, Ansell J, Deykin D (2001) Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 119:8S–21S
Hirsh J, Fuster V, Ansell J, Halperin JL (2003) American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. J Am Coll Cardiol 41:1633–1652
Herman D, Locatelli I, Grabnar I, Peternel P, Stegnar M, Mrhar A, Breskvar K, Dolzan V (2005) Influence of CYP2C9 polymorphisms, demographic factors and concomitant drug therapy on warfarin metabolism and maintenance dose. Pharmacogenomics J 5:193–202
Ghosal A, Lu X, Penner N, Gao L, Ramanathan R, Chowdhury SK, Kishnani NS, Alton KB (2011) Identification of human liver cytochrome P450 enzymes involved in the metabolism of SCH 530348 (Vorapaxar), a potent oral thrombin protease-activated receptor 1 antagonist. Drug Metab Dispos 39:30–38
Shanmugam S, Lee ES, Jeong TC, Yong CS, Choi HG, Woo JS, Yoo BK (2007) The effect of 1-furan-2-yl-3-pyridine-2-yl-propenone on pharmacokinetic parameters of warfarin. Arch Pharm Res 30:898–904
Kosoglou T, Reyderman L, Kasserra C, Jennings LK, Young S, Xuan F, Pei J, Maxwell SE, Schiller J, Meehan AG et al (2012) No differences in the pharmacodynamics and pharmacokinetics of the thrombin receptor antagonist vorapaxar between healthy Japanese and Caucasian subjects. Eur J Clin Pharmacol 68:291–300
Lai AA, Levy RH, Cutler RE (1978) Time-course of interaction between carbamazepine and clonazepam in normal man. Clin Pharmacol Ther 24:316–323
Leucuta SE, Vlase L (2006) Pharmacokinetics and metabolic drug interactions. Curr Clin Pharmacol 1:5–20
Jacobs T, De Ridder F, Rusch S, Van Peer A, Molenberghs G, Bijnens L (2008) Including information on the therapeutic window in bioequivalence acceptance. Pharm Res 25:2628–2638
Johnston M, Harrison L, Moffat K, Willan A, Hirsh J (1996) Reliability of the international normalized ratio for monitoring the induction phase of warfarin: comparison with the prothrombin time ratio. J Lab Clin Med 128:214–217
Kirkwood TB (1983) Calibration of reference thromboplastins and standardisation of the prothrombin time ratio. Thromb Haemost 49:238–244
Reyderman L, Kosoglou T, Tseng J, Xuan F, Schiller J, Cutler DL, Kim K (2011) The effect of food and antacid on pharmacokinetics (PK) of SCH 530348 in healthy subjects. Clin Pharmacol Ther 85 [Suppl 1]:S21
Acknowledgements
The authors wish to thank the subjects for their participation in this study. The authors also wish to thank Kathleen Newcomb (Merck Sharp & Dohme Corp.) for her expert assistance with the preparation of this manuscript for publication.
Conflict of interest
T. Kosoglou, Y. Zhu, F. Xuan, L. Black, A.O. Johnson-Levonas, M. Martinho, P. Statkevich, and D.L. Cutler all declare that they are/were full-time employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA, at the time of the study and may own stock or hold stock options in the company. This study was funded by Schering-Plough Corporation (now Merck & Co., Inc., Whitehouse Station, NJ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kosoglou, T., Zhu, Y., Xuan, F. et al. Vorapaxar, an oral PAR-1 receptor antagonist, does not affect the pharmacokinetics and pharmacodynamics of warfarin. Eur J Clin Pharmacol 68, 1509–1516 (2012). https://doi.org/10.1007/s00228-012-1271-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1271-0