Abstract
Purpose
The objective of this study was to develop a population pharmacokinetic model and investigate the effect of several demographic covariates on metformin pharmacokinetics in patients with type 2 diabetes mellitus, over a wide range of weights.
Methods
A total of 105 patients received different metformin regimens, and pharmacokinetic sampling included a minimum of two concentrations per patient. Plasma determination of metformin was assayed by high performance liquid chromatography. Population pharmacokinetics was modelled using a nonlinear mixed effects model program (Monolix version 3.1 s).
Results
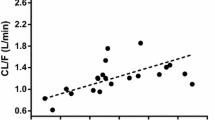
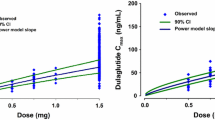
An open one-compartment model adequately described metformin data. Lean body weight was a better size descriptor than actual body weight or ideal body weight for clearance (CL/F) and volume (V/F) parameters. CL/F was negatively related to age and serum creatinine (SCr). The estimation of specific coefficients for these effects gave better results than the use of renal function descriptors (Cockroft or MDRD). A dose effect in the relative bioavailability was demonstrated.
Conclusion
The pharmacokinetics of metformin was influenced by lean body weight on an allometric basis and was related to markers of renal function, age, and serum creatinine in this population of 105 patients.




Similar content being viewed by others
References
Nathan DM, Buse JB, Davidson MB et al (2009) Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care 32:193–203
Hanley MJ, Abernethy DR, Greenblatt DJ (2010) Effect of obesity on the pharmacokinetics of drugs in human. Clin Pharmacokinet 49:71–87
Cheymol G (2000) Effects of obesity on pharmacokinetics. Clin Pharmacokinet 39:215–231
Sparreboom A, Wolff AC, Mathijssen RHJ et al (2010) Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. J Clin Oncol 25:4707–4713
Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL (1997) Efficacy of metformin in type II diabetes: results of a double-blind, placebo controlled, dose response trial. Am J Med 103:491–497
Graham GG, Punt J, Arora M et al (2011) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50:81–98
Devine D (1974) Case study number 25 gentamicin therapy. Drug Intell Clin Pharm 8:650–655
Janmahasatian S, Dufull SB, Ash S et al (2005) Quantification of lean bodyweight. Clin Pharmacokinet 44:1051–1065
Amini H, Ahmadiani A, Gazerani P (2005) Determination of metformin in human plasma by high-performance liquid chromatography. J Chrom B 824:319–322
Kuhn E, Lavielle M (2005) Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal 49:1020–1030
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
McLeay S, Morrish G, Kirkpatrick C et al (2009) Encouraging the move towards predictive population models for the obese using propofol as a motivating example. Pharm Res 26:1626–1634
Mould D, Holford N, Schellens J et al (2002) Population pharmacokinetic and adverse event analysis of topotecan in patients with solid tumors. Clin Pharmacol Ther 71:334–348
Sirtori CR, Franceschini G, Galli-Kienle M et al (1978) Disposition of metformin (N, N-dimethylbiguanide) in man. Clin Pharmacol Ther 24:683–693
Pentikaïnen PJ, Neuvonen PJ, Pentillä A (1979) Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol 16:195–202
Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF (1981) Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol 12:235–246
Scheen AJ (1996) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 30:359–371
Hong Y, Rohatagi S, Habtemariam B, Walker J, Schwartz SL, Mager DE (2008) Population exposure-response modeling of metformin in patients with type 2 diabetes mellitus. J Clin Pharmacol 48:696–707
Schäfer G (1983) Biguanides: a review of history, pharmacodynamics and therapy. Diab Metab 9:148–163
Han PY, Duffull SB, Kirkpatrick CM, Green B (2007) Dosing in obesity: a simple solution to a big problem. Clin Pharmacol Ther 82:505–508
Erstadt BL (2004) Dosing of medications in morbidly obese patients in intensive care unit setting. Intensive Care Med 30:18–32
Traynor AM, Nafziger AN, Bertino JS (1995) Aminoglycoside dosing weight correction factors for patients of various body sizes. Antimicrob Agents Chemother 39:545–548
Green B, Duffull SB (2004) What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol 58:119–133
Sambol NC, Chiang J, Lin ET et al (1995) Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol 35:1094–1102
Lalau JD, Vermersch A, Hary L, Andrejak M, Isnard F, Quichaud J (1990) Type 2 diabetes in the elderly: an assessment of metformin. Int J Clin Pharmacol Ther Toxico 28:329–332
Karim A, Slater M, Bradford D et al (2007) Oral antidiabetic drugs: bioavailability assessment of fixed-dose combination tablets of pioglitazone and metformin. Effect of body weight, gender, and race on systematic exposures of each drug. J Clin Pharmacol 47:37–47
Sambol NC, Brookes LG, Chiang J et al (1996) Food intake and dosage level, but not tablet vs solution dosage form, affect the absorption of metformin HCl in man. Br J Clin Pharmacol 42:510–512
Tzvetkov MV, Vormfelde SV, Balen B et al (2009) The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2 and OCT3 on the renal clearance of metformin. Clin Pharmacol Ther 86:299–306
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bardin, C., Nobecourt, E., Larger, E. et al. Population pharmacokinetics of metformin in obese and non-obese patients with type 2 diabetes mellitus. Eur J Clin Pharmacol 68, 961–968 (2012). https://doi.org/10.1007/s00228-011-1207-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1207-0