Abstract
Objective
Chlorproguanil (CPG)–dapsone (DDS)−artesunate was in development for the treatment of uncomplicated Plasmodium falciparum malaria. The pharmacokinetics of CPG, DDS, artesunate and their metabolites chlorcycloguanil (CCG), monoacetyl dapsone (MADDS) and dihydroartemisinin (DHA) were investigated in patients with P. falciparum given CPG−DDS alone or plus artesunate.
Methods
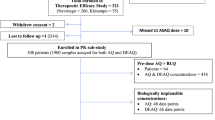
Adult patients from Malawi and The Gambia taking part in a phase II clinical trial were randomised to receive a 3-day treatment of CPG–DDS alone (2/2.5 mg/kg/day) or plus 1, 2 or 4 mg/kg/day artesunate. Blood samples for pharmacokinetic analysis were collected up to 24 h post–first dose.
Results
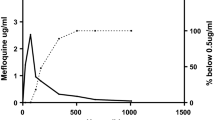
The pharmacokinetic analysis included 115 patients. For CPG, there was no significant effect of artesunate on Cmax or AUC(0–24), except the 90% confidence interval (CI) for AUC(0−24) for the 4 mg/kg artesunate dose was slightly below that for the standard bioequivalence range (90% CI 0.78, 1.11); this was not considered clinically relevant. Artesunate increased the CCG AUC(0−24) by 6−17% and Cmax by 0−16%. Artesunate had no significant effect on the rate or extent of absorption of DDS. For MADDS, artesunate increased the AUC(0−24) by 13−47% and Cmax by 8−45%. For 1, 2 and 4 mg/kg artesunate dosing, artesunate AUC(0−∞) was 64.6, 151 and 400 ng·h/ml and Cmax 48.9, 106 and 224 ng/ml respectively; DHA AUC(0−∞) was 538, 1,445 and 3,837 ng·h/ml and Cmax 228, 581 and 1,414 ng/ml respectively. Using a power model, the point estimates of slope were greater than 1 for artesunate AUC(0−t) by 16% and Cmax by 5% and for DHA by 39 and 21% respectively.
Conclusion
Artesunate did not significantly affect CPG or DDS pharmacokinetics. For CCG and MADDS, small to moderate increases in exposure with artesunate dosing were observed. There was a greater than proportional increase in artesunate and DHA exposure with increasing artesunate dose. These effects are not considered to be clinically relevant. It should be noted that the CPG−DDS−artesunate programme has now been stopped following unacceptable haematological toxicity in patients with glucose-6-phosphate dehydrogenase deficiency during a phase III trial. In addition, the CPG−DDS combination has been withdrawn from clinical use.



Similar content being viewed by others
Abbreviations
- CPG:
-
Chlorproguanil
- CCG:
-
Chlorcycloguanil
- DDS:
-
Dapsone
- DHA:
-
Dihydroartemisinin
- MADDS:
-
Monoacetyl dapsone
References
World Health Organisation (2005) World malaria report. World Health Organisation, Geneva. http://rbm.who.int/wmr2005/. Accessed, 23 August 2008
Sachs J, Malaney P (2002) The economic and social burden of malaria. Nature 415:680–685
Hyde JE (2005) Drug-resistant malaria. Trends Parasitol 21:494–498
Alin MH, Bjorkman A (1994) Concentration and time dependency of artemisinin efficacy against Plasmodium falciparum in vitro. Am J Trop Med Hyg 50:771–776
Angus BJ, Thaiaporn I, Chanthapadith K, Suputtamongkol Y, White NJ (2002) Oral artesunate dose-response relationship in acute falciparum malaria. Antimicrob Agents Chemother 46:778–782
Price RN, Nosten F, Luxemburger C, van Vugt M, Phaipun L, Chongsuphajaisiddhi T, White NJ (1997) Artesunate/mefloquine treatment of multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg 91:574–577
van Vugt M, Looareesuwan S, Wilairatana P, McGready R, Villegas L, Gathmann I, Mull R, Brockman A, White NJ, Nosten F (2000) Artemether-lumefantrine for the treatment of multidrug-resistant falciparum malaria. Trans R Soc Trop Med Hyg 94:545–548
Teja-Isavadharm P, Watt G, Eamsila C, Jongsakul K, Li Q, Keeratithakul G, Sirisopana N, Luesutthiviboon L, Brewer TG, Kyle DE (2001) Comparative pharmacokinetics and effect kinetics of orally administered artesunate in healthy volunteers and patients with uncomplicated falciparum malaria. Am J Trop Med Hyg 65:717–721
Newton P, Suputtamongkol Y, Teja-Isavadharm P, Pukrittayakamee S, Navaratnam V, Bates I, White N (2000) Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob Agents Chemother 44:972–977
Suputtamongkol Y, Newton PN, Angus B, Teja-Isavadharm P, Keeratithakul D, Rasameesoraj M, Pukrittayakamee S, White NJ (2001) A comparison of oral artesunate and artemether antimalarial bioactivities in acute falciparum malaria. Br J Clin Pharmacol 52:655–661
Winstanley P (2001) Chlorproguanil-dapsone (LAPDAP) for uncomplicated falciparum malaria. Trop Med Int Health 6:952–954
Alloueche A, Bailey W, Barton S, Bwika J, Chimpeni P, Falade CO, Fehintola FA, Horton J, Jaffar S, Kanyok T et al (2004) Comparison of chlorproguanil-dapsone with sulfadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria in young African children: double-blind randomised controlled trial. Lancet 363:1843–1848
Simpson JA, Hughes D, Manyando C, Bojang K, Aarons L, Winstanley P, Edwards G, Watkins WA, Ward S (2006) Population pharmacokinetic and pharmacodynamic modelling of the antimalarial chemotherapy chlorproguanil/dapsone. Br J Clin Pharmacol 61:289–300
Winstanley P, Watkins W, Muhia D, Szwandt S, Amukoye E, Marsh K (1997) Chlorproguanil/dapsone for uncomplicated Plasmodium falciparum malaria in young children: pharmacokinetics and therapeutic range. Trans R Soc Trop Med Hyg 91:322–327
Watkins WM, Mberu EK, Winstanley PA, Plowe CV (1997) The efficacy of antifolate antimalarial combinations in Africa: a predictive model based on pharmacodynamic and pharmacokinetic analyses. Parasitol Today 13:459–464
Nzila AM, Nduati E, Mberu EK, Hopkins Sibley C, Monks SA, Winstanley PA, Watkins WM (2000) Molecular evidence of greater selective pressure for drug resistance exerted by the long-acting antifolate pyrimethamine/sulfadoxine compared with the shorter-acting chlorproguanil/dapsone on Kenyan Plasmodium falciparum. J Infect Dis 181:2023–2028
Sulo J, Chimpeni P, Hatcher J, Kublin JG, Plowe CV, Molyneux ME, Marsh K, Taylor TE, Watkins WM, Winstanley PA (2002) Chlorproguanil-dapsone versus sulfadoxine-pyrimethamine for sequential episodes of uncomplicated falciparum malaria in Kenya and Malawi: a randomised clinical trial. Lancet 360:1136–1143
Tiono A, Dicko A, Ndububa D, Abenyega T, Pitmang S, Awobusuyi J, Pamba A, Duparc S, Goh L-E, Harrell E et al (2008) Chlorproguanil-dapsone-artesunate vs. chlorproguanil-dapsone: a randomised, double-blind phase III trial for the treatment of acute uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg 79 (6)(Suppl):172
Premji Z, Umeh R, Owusu-Agyei S, Esamai F, Ezedinachi E, Oguche S, Borrmann S, Sowunmi A, Duparc S, Kirby P et al (2008) Chlorproguanil-dapsone-artesunate vs. artemether-lumefantrine: a randomised, double-blind phase III trial for the treatment of acute, uncomplicated Plasmodium falciparum malaria in African children and adolescents. Am J Trop Med Hyg 79 (6)(Suppl):225
Wootton DG, Opara H, Biagini GA, Kanjala MK, Duparc S, Kirby PL, Woessner M, Neate C, Nyirenda M, Blencowe H et al (2008) Open-label comparative clinical study of chlorproguanil-dapsone fixed dose combination (Lapdap) alone or with three different doses of artesunate for uncomplicated Plasmodium falciparum malaria. PLoS ONE 3:e1779
U.S. Drug and Food Administration (2002) Bioavailability and bioequivalence studies for orally administered drug products - general considerations. U.S. Food and Drug Administration Center for Drug Evaluation and Research, Washington DC. http://www.fda.gov/cder/guidance/4964dft.pdf. Accessed 1 October 2007
Gough K, Hutchison M, Keene O, Byrom B, Ellis S, Lacey L, Mckellar J (1995) Assessment of dose proportionality - report from the Statisticians in the Pharmaceutical Industry/Pharmacokinetic UK Joint Working Party. Drug Inf J 29:1039–1048
Wright JD, Helsby NA, Ward SA (1995) The role of S-mephenytoin hydroxylase (CYP2C19) in the metabolism of the antimalarial biguanides. Br J Clin Pharmacol 39:441–444
Asimus S, Elsherbiny D, Hai TN, Jansson B, Huong NV, Petzold MG, Simonsson US, Ashton M (2007) Artemisinin antimalarials moderately affect cytochrome P450 enzyme activity in healthy subjects. Fundam Clin Pharmacol 21:307–316
Zuidema J, Hilbers-Modderman ES, Merkus FW (1986) Clinical pharmacokinetics of dapsone. Clin Pharmacokinet 11:299–315
Tingle MD, Mahmud R, Maggs JL, Pirmohamed M, Park BK (1997) Comparison of the metabolism and toxicity of dapsone in rat, mouse and man. J Pharmacol Exp Ther 283:817–823
Winter HR, Wang Y, Unadkat JD (2000) CYP2C8/9 mediate dapsone N-hydroxylation at clinical concentrations of dapsone. Drug Metab Dispos 28:865–868
Ilett KF, Ethell BT, Maggs JL, Davis TM, Batty KT, Burchell B, Binh TQ, le Thu TA, Hung NC, Pirmohamed M et al (2002) Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP-glucuronosyltransferases. Drug Metab Dispos 30:1005–1012
Diem Thuy LT, Ngoc Hung L, Danh PT, Na-Bangchang K (2008) Absence of time-dependent artesunate pharmacokinetics in healthy subjects during 5-day oral administration. Eur J Clin Pharmacol 64:993–998
Herrlin K, Massele AY, Rimoy G, Alm C, Rais M, Ericsson O, Bertilsson L, Gustafsson LL (2000) Slow chloroguanide metabolism in Tanzanians compared with white subjects and Asian subjects confirms a decreased CYP2C19 activity in relation to genotype. Clin Pharmacol Ther 68:189–198
van Vugt M, Edstein MD, Proux S, Lay K, Ooh M, Looareesuwan S, White NJ, Nosten F (1999) Absence of an interaction between artesunate and atovaquone–proguanil. Eur J Clin Pharmacol 55:469–474
Gelber R, Peters JH, Gordon GR, Glazko AJ, Levy L (1971) The polymorphic acetylation of dapsone in man. Clin Pharmacol Ther 12:225–238
Price R, van Vugt M, Phaipun L, Luxemburger C, Simpson J, McGready R, ter Kuile F, Kham A, Chongsuphajaisiddhi T, White NJ et al (1999) Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives. Am J Trop Med Hyg 60:547–555
Batty KT, Thu LT, Davis TM, Ilett KF, Mai TX, Hung NC, Tien NP, Powell SM, Thien HV, Binh TQ et al (1998) A pharmacokinetic and pharmacodynamic study of intravenous vs oral artesunate in uncomplicated falciparum malaria. Br J Clin Pharmacol 45:123–129
Binh TQ, Ilett KF, Batty KT, Davis TM, Hung NC, Powell SM, Thu LT, Thien HV, Phuong HL, Phuong VD (2001) Oral bioavailability of dihydroartemisinin in Vietnamese volunteers and in patients with falciparum malaria. Br J Clin Pharmacol 51:541–546
Khanh NX, de Vries PJ, Ha LD, van Boxtel CJ, Koopmans R, Kager PA (1999) Declining concentrations of dihydroartemisinin in plasma during 5-day oral treatment with artesunate for falciparum malaria. Antimicrob Agents Chemother 43:690–692
Lindegardh N, Hanpithakpong W, Kamanikom B, Singhasivanon P, Socheat D, Yi P, Dondorp AM, McGready R, Nosten F, White NJ et al (2008) Major pitfalls in the measurement of artemisinin derivatives in plasma in clinical studies. J Chromatogr B Analyt Technol Biomed Life Sci 876:54–60
Acknowledgements
Naomi Richardson of Magenta Communications Ltd developed a first draft of this paper based on the study report approved by the investigators, collated author contributions to the manuscript and was funded by GlaxoSmithKline (GSK). The authors wish to thank the study participants and the site and GSK staff involved in the conduct of this study and staff in World Wide Bioanalysis at GSK, in particular, Alex Georgiou, Wayne Wright and Matthew Barfield.
Conflict of interest
Ann K. Miller and Paula Kirby are current employees of GlaxoSmithKline. Stephan Duparc is a former employee of GlaxoSmithKline and a current employee of Medicines for Malaria Venture. Nibedita Bandyopadhyay is a former employee of GlaxoSmithKline and a current employee of Johnson & Johnson, Raritan, NJ, USA.
Funding
The development of chlorproguanil–dapsone–artesunate fixed dose combination is by public–private partnership and was commissioned by the Medicines for Malaria Venture. A development agreement was made between the UNICEF – United Nations Development Programme – World Bank – World Health Organisation Special Programme for Research and Training in Tropical Medicine and GlaxoSmithKline PLC. The UK government Department for International Development supplied some initial funding to the project. Liverpool School of Tropical Medicine, Liverpool University, and the London School of Hygiene and Tropical Medicine joined the joint development team as academic partners. A committee comprising representatives from all development partners developed the protocol.
Trial registration
Clinicaltrials.gov (http://clinicaltrials.gov/ct/show/NCT00519467?order=6) identifier NCT00519467; study ID number: SB-714703/003.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miller, A.K., Bandyopadhyay, N., Wootton, D.G. et al. Pharmacokinetics of chlorproguanil, dapsone, artesunate and their major metabolites in patients during treatment of acute uncomplicated Plasmodium falciparum malaria. Eur J Clin Pharmacol 65, 977–987 (2009). https://doi.org/10.1007/s00228-009-0672-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-009-0672-1