Abstract
Objective
To evaluate the effect of publicity surrounding the Women’s Health Initiative (WHI) and Million Women (MW) studies on prescribing of all hormone replacement therapy (HRT) preparations and bisphosphonates in Ireland.
Methods
The General Medical Services (GMS) prescription database was used to identify the study population. Prescriptions were identified for HRT and bisphosphonate preparations [using WHO Anatomical Therapeutic Chemical (ATC) classification codes] in female patients aged 45–69 years in Ireland during a 4-year study period (January 2001–December 2004). Prescription rates were calculated monthly. Prevalence and incidence of HRT use was examined.
Results
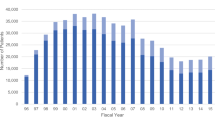
There was a significant reduction in prevalence for all HRT preparations following the WHI trial (test for change in trend p<0.0001), which persisted after the MW study. The incidence of combined oestrogen/progestogen HRT declined after the WHI trial (test for change in trend p=0.004). Bisphosphonate prescribing showed a significant increase throughout the study period (p<0.0001).
Conclusion
The findings suggest that coverage surrounding the publication of clinical trials appears to have had a negative impact on the rate of HRT prescribing. The findings regarding the coincident increase in use of bisphosphonates may suggest that prescribers and users were less likely to regard HRT as an appropriate therapy in the management of osteoporosis for some time before guidance was issued by the regulatory authorities.


Similar content being viewed by others
References
Writing Group for the Women’s Health Initiative Investigators (2002) Risks and benefits of estrogen plus progestogen in healthy postmenopausal women. JAMA 288:321–333
Hersh A, Stefanick M, Stafford R (2004) National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA 291:47–53
Haas J, Kaplan C, Gerstenberger E, Kerlikowske K (2004) Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med 140:184–188
Million Women Study Collaborators (2003) Breast cancer and hormone replacement therapy in the Million Women Study. Lancet 362:419–427
Annual Report (2003) General Medical Services (Payments) Board, Dublin
Feely J, Chan R, McManus J, O’Shea B (1999) The influence of hospital-based prescribers on prescribing in general practice. Pharmacoeconomics 16:175-181
ATC index with DDDs (2003) WHO Collaborating Centre for Drug Statistics Methodology, Oslo, Norway
Williams D, Feely J (2001) Pharmacoepidemiology–an Irish perspective. Pharmacoepidemiol Drug Saf 10:641–645
Wagner A, Soumerai F, Zhang F, Ross-Degnan D (2002) Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 27:299–309
Hormone replacement therapy (2002) Irish Medicines Board Drug Safety Newsletter July 15: 1–2. Available at http://www.imb.ie
Royal College of Physicians Edinburgh (2003) Consensus conference on hormone replacement therapy: Final consensus statement. October.http://www.rcpe.ac.ul/esd/consensus/hrt_03.html
Hormone replacement therapy (2003) Risk/benefit unfavourable for first-line use for prevention of osteoporosis. Irish Medicines Board Drug Safety Newsletter Dec 18: 1–3. Available at http://www.imb.ie
Bastian LA, Smith CM, Nanda K (2003) Is this woman perimenopausal? JAMA 289:895–902
Rymer J, Wilson R, Ballard K (2003) Making decisions about hormone replacement therapy. BMJ 326:322–326
Stearns V, Ullmer L, Lopez JF et al (2002) Hot flushes. Lancet 360:1851–1861
Fallon U, Kelleher C (2000) Hormone replacement therapy: a survey of Irish general practitioners. Ir Med J 93(1):10–14
Bestul M, McCollum M, Hansen L, Saseen J (2004) Impact of the women’s health initiative trial results on hormone replacement therapy. Pharmacotherapy 24(4):495–499
Townsend J, Nanchahal K (2005) Hormone replacement therapy: limited response in the UK to the new evidence. Br J Gen Pract 55:555
Acknowledgement
We would like to thank the Irish GMS (Payments) Board for providing us with the data on which this study is based and the Health Research Board of Ireland, for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Usher, C., Teeling, M., Bennett, K. et al. Effect of clinical trial publicity on HRT prescribing in Ireland. Eur J Clin Pharmacol 62, 307–310 (2006). https://doi.org/10.1007/s00228-005-0083-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0083-x