Abstract
Summary
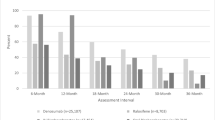
Among 125,954 new users of osteoporosis (OP) medications, 77 % of subjects stopped OP medications, and 23 % of subjects added or started a new OP medication during follow-up, with the first addition or start of a new OP medication occurring in a mean of 739 days after original OP treatment.
Introduction
We described patterns and predictors of OP medication use, focusing on treatment changes over time.
Methods
We analyzed health and pharmacy insurance claims for a large cohort of low-income Medicare beneficiaries with a drug benefit for the years 1998–2008. Study subjects had documented Medicare claims and no receipt of OP medications (i.e., bisphosphonate, raloxifene, calcitonin, teriparatide, or hormonal therapy) during a baseline of 180 days. Subjects were then required to start an OP medication. Baseline patient and prescriber characteristics were assessed in multivariable Cox regression models to identify correlates of adding or starting a new OP medication. Fractures, bone mineral density testing, and visits with endocrinologists or rheumatologists occurring after baseline were also examined as correlates.
Results
We included 125,954 new users of OP medications with a mean age of 78 years, 97 % female, and 92 % white. OP medication prescribers included specialists (i.e., endocrinologists or rheumatologists) (6.2 %), orthopedic surgeons (1.0 %), primary care providers (64.9 %), other physicians (3.7 %), and missing (24.1 %). Seventy-seven percent of subjects stopped OP medications, and 23 % of subjects added or started a new OP medication during follow-up, with the first addition or start of a new OP medication occurring in a mean of 739 days after original OP treatment; 4 % added or started a new OP medication more than once. In fully adjusted models, many baseline variables correlated with starting a second OP medication. Post-baseline fractures [hazard ratio (HR) 1.76, 95 % confidence interval (CI) 1.71–1.82] and bone mineral density testing (HR 2.94, 95 % CI 2.86–3.03) were strong predictors.
Conclusion
Approximately one quarter of patients starting an OP medication added or started a new OP medication during follow-up. Long-term sequential treatment strategy trials would inform optimal medication treatment for OP.
Similar content being viewed by others
References
Gass M, Dawson-Hughes B (2006) Preventing osteoporosis-related fractures: an overview. Am J Med 119(4 Suppl 1):S3–S11
Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C et al (2002) Meta-analyses of therapies for postmenopausal osteoporosis. IX: summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev 23(4):570–8
Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V et al (2008) Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev 1, CD001155
National Osteoporosis Foundation (2013) Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation, Washington
Stafford RS, Drieling RL, Hersh AL (2004) National trends in osteoporosis visits and osteoporosis treatment, 1988–2003. Arch Intern Med 164(14):1525–30
Lewiecki EM, Adler RA, Bilezikian JP, Bouxsein ML, Marcus R, McClung MR, et al. Osteoporosis update from the 2012 Santa Fe bone symposium. J Clin Densitom 2013.
Solomon DH, Rekedal L, Cadarette SM (2009) Osteoporosis treatments and adverse events. Curr Opin Rheumatol 21(4):363–8
Solomon DH, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM et al (2005) Compliance with osteoporosis medications. Arch Intern Med 165(20):2414–9
Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ (2007) Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc 82(12):1493–501
Xu Y, Viswanathan HN, Ward MA, Clay B, Adams JL, Stolshek BS et al (2013) Patterns of osteoporosis treatment change and treatment discontinuation among commercial and Medicare Advantage Prescription Drug members in a national health plan. J Eval Clin Pract 19(1):50–9
Schisterman EF, Cole SR, Platt RW (2009) Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 20(4):488–95
Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S (2011) A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 64:749–59
Brookhart MA, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, Patrick AR, Mogun H, Solmon DH (2007) Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med 120(3):251–256
Cummings SR, Cosman F, Eastell R, Reid IR, Mehta M, Lewiecki EM (2013) Goal-directed treatment of osteoporosis. J Bone Miner Res 28(3):433–8
Employee Benefits Research Institute (2007) Income of the elderly population age 65 and over; May
Funding
This paper was funded by Amgen.
Conflicts of interest
Dr. Solomon receives salary support from research support to Brigham and Women’s Hospital from Amgen, Lilly, and CORRONA. He serves in unpaid roles on two trials outside of osteoporosis, one funded by Pfizer and the other by Novartis. He has provided unpaid consultative services to Lilly and Bristol Myers Squibb. He receives royalties from UpToDate. Dr. Chandler, Dr. Bower, and Dr. Barron were all employees of Amgen at the time of this project. Erika Brown, Helen Mogun, and Jessica Myers Franklin declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
List of osteoporosis treatments
Record of at least one pharmacy claim for:
-
Oral bisphosphonates: alendronate (10 mg po per day or 70 mg po per week), risedronate (5 mg po per day, 35 mg po per week, or 150 mg po per month), or ibandronate (150 mg po per month).
-
Raloxifene (60 mg po per day)
-
Calcitonin (200 IU intranasal spray per day)
-
Hormone replacement therapy: oral and patches
-
IV bisphosphonates: zoledronic acid (measured as an infusion in medical claims J3487 or Q4095)
-
Teriparatide (20 μg injectable per day).
Appendix 2
Rights and permissions
About this article
Cite this article
Solomon, D.H., Brown, E.M., Chandler, D. et al. Patterns of treatment among a cohort of older low-income adults starting new medications for osteoporosis. Osteoporos Int 25, 2255–2262 (2014). https://doi.org/10.1007/s00198-014-2757-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2757-7