Abstract
In this study, the annual movements of a seabird species, the red-throated diver (Gavia stellata), were investigated in space and time. Between 2015 and 2017, 33 individuals were fitted with satellite transmitters at the German Bight (eastern North Sea). In addition, stable isotope analyses of feathers (δ13C) were used to identify staging areas during the previous moult. The German Bight is an important area for this species, but is also strongly affected by anthropogenic impacts. To understand how this might affect populations, we aimed to determine the degree of connectivity and site fidelity, and the extent to which seasonal migrations vary among different breeding locations in the high Arctic. Tagged individuals migrated to Greenland (n = 2), Svalbard (n = 2), Norway (n = 4) and northern Russia (n = 25). Although individuals from a shared breeding region (northern Russia) largely moved along the same route, individuals dispersed to different, separate areas during the non-breeding phase. Kernel density estimates also overlapped only partially, indicating low connectivity. The timing of breeding was correlated with the breeding longitude, with 40 days later arrival at the easternmost than westernmost breeding sites. Repeatability analyses between years revealed a generally high individual site fidelity with respect to spring staging, breeding and moulting sites. In summary, low connectivity and the distribution to different sites suggests some resilience to population decline among subpopulations. However, it should be noted that the majority of individuals breeding in northern Russia migrated along a similar route and that disturbance in areas visited along this route could have a greater impact on this population. In turn, individual site fidelity could indicate low adaptability to environmental changes and could lead to potential carry-over effects. Annual migration data indicate that conservation planning must consider all sites used by such mobile species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Migratory birds are increasingly affected by environmental changes, disturbances and threats along their migration routes (Wilcove and Wikelski 2008). Therefore, information about annual movements is important for effective conservation and management (Marra et al. 2018; Johnston et al. 2020). Many species, such as seabirds, are long-lived with delayed sexual maturity and low annual reproductive rates (Schreiber and Burger 2001). Survival of adult birds, as well as reproductive success, therefore, influence their population growth rates (Sæther and Bakke 2000). Different migratory strategies, including different winter locations, can influence fitness differently, through variable energy costs and winter habitat conditions (Alves et al. 2013). Altered environmental conditions in the stationary non-breeding periods can affect migratory timing or carry-over to affect survival and reproduction in the breeding areas (Marra et al 1998; Harrison et al. 2011; Winkler et al. 2014; Rushing et al. 2016). Information about the site use of migratory birds throughout the whole annual cycle and their ability to respond to environmental change is, therefore, important to conserve such long-lived and mobile species (Moore et al. 2005; Runge et al. 2014).
Migratory connectivity, i.e. the degree to which individuals from one breeding or wintering population stay together and use similar sites along the annual cycle, provides essential information to assess the impact of environmental change in habitats along the migratory route (Webster et al. 2002; Martin et al. 2007). The degree of migratory connectivity determines to which extent different breeding populations experience similar non-breeding conditions (Esler 2000; Taylor and Norris 2010; Cresswell 2014; Finch et al. 2017). Migratory connectivity is defined along a continuum from strong connectivity (low interpopulation spread and use of population-specific non-breeding areas) to a low or diffuse connectivity (high interpopulation spread) (Webster et al. 2002; Newton 2008; Finch et al. 2017). In a low connectivity scenario, individuals from a given breeding population may mix with individuals from other breeding regions during the non-breeding season (Finch et al 2017). Recent studies (i.e. Gilroy et al. 2016) introduced further terms such as migratory diversity which expresses the within-population variability in migratory movements and suggest that migratory diversity may help to facilitate species responses to environmental change.
To understand how migrants might be affected by environmental change in breeding and non-breeding sites, we need to understand the migratory pattern in space and time such as the variation in migration duration, number of staging stops and temporal pattern among the different migration routes and breeding sites. These baseline measurements are necessary to monitor future shifts in timing or to identify carry-over effects (Gordo 2007; Studds et al. 2008; Duijns et al. 2017). Migratory movements and their timings can be linked with geographic position, resource allocation or climatic variables on the breeding areas (Conklin 2010). Climatic conditions on the wintering grounds can also affect migratory timing, with some species responding to milder winters with earlier arrival on the breeding grounds (Gunnarson and Tómasson 2011). The total duration of migration and thus the arrival time at the destination may also depend on the conditions at stop-over sites, which influence the decision to stay or continue (Weber and Houston 1997; Klinner et al. 2020). Local resource availability and competition at moulting, wintering or stop-over habitats are important for migratory movements (Kokko 1999; Moore 2005; Kölzsch et al. 2016; Fayet et al. 2017). Reduced habitat quality at these locations could result in delayed or extended stays and altered timing of annual movements (Marra et al. 1998).
Individual fidelity towards sites used throughout the year, as well as temporal repeatability, could also be important for predicting the response of migrants to environmental change. Individual behavioural consistency might determine how individuals respond to environmental change and how much populations could be affected by habitat changes and possible carry-over effects (Reed et al. 2009; Dias et al. 2010). Individuals with a high site fidelity may be less flexible to voluntarily change sites and more sensitive to displacement caused by disturbance. Consequently, less flexible individuals might adapt more slowly to a new environment, than an individual that is familiar with multiple sites and can use flexible strategies (Catry et al. 2004; McFarlane et al. 2014; Merkel et al. 2021). Individual site utilisation and movements within and between years are, therefore, important to consider in conservation decisions (Croxall et al. 2005; González-Solís et al. 2007).
In this study, we analysed the migratory behaviour of a seabird species, the red-throated diver (Gavia stellata), that is increasingly influenced by human activities in one of their most important winter and spring staging areas in Europe, the German Bight (eastern North Sea) (Garthe et al. 2007, 2015; Dierschke et al. 2012; Burger et al. 2019; Mendel et al. 2019; Heinänen et al. 2020). In this winter population, strong avoidance of offshore wind farm areas was observed (Mendel et al. 2019; Heinänen et al. 2020; Vilela et al. 2021) but, so far, no decline in wintering population numbers of this long-lived species (Vilela et al. 2021). Red-throated divers are listed in Annex II of the Bern Convention, Annex I of the EU Birds Directive and as critically endangered on the HELCOM (Helsinki Commission) convention (BirdLife International 2022). The species is widespread in the Holarctic, with breeding areas in the Arctic tundra regions north of 60° latitude and wintering areas in temperate coastal ocean waters. Breeding populations in Shetland, Sweden, Finland and Greenland have been linked with wintering areas such as the Baltic Sea, Skagerrak, the North Sea and further south to the Bay of Biscay (Cramp and Simmons 1977; Okill 1994; Wetlands International 2019). Ring recoveries suggest that younger birds move further south during winter than older birds (Okill 1994).
Moult is one of the three main energy-demanding events in the annual cycle of birds and usually occurs at a different time from breeding and migration (Newton 2009, 2011). Information about space use in this sensitive period of the year is important for conservation measures, but little is known about the temporal and spatial patterns of moult in red-throated divers. To date, it is known that red-throated divers moult their wing feathers simultaneously, rendering them temporarily flightless. Wing moult takes place in autumn (August to November, Stresemann and Stresemann 1966), in an area that is visited after leaving the breeding area. Recoveries of dead birds washed up on the coast in the North and Baltic Sea have shown that the birds moult their wing feathers in this period and also change from breeding to winter plumage (Berndt and Drenckhahn 1974, Mendel et al. 2008). In the following spring, birds moult their body feathers back into the breeding plumage (Stresemann and Stresemann 1966).
We used satellite telemetry which has been shown to be highly suitable to study migratory movements and spatial–temporal patterns of red-throated divers within and between years (Schmutz 2014; Paruk et al. 2015; Spiegel et al. 2017; McCloskey et al. 2018). Additionally, we used carbon stable isotope analyses of neck feathers in combination with satellite tracking data to infer moulting areas used prior to capture. Stable isotope values of a predator are related to those of its prey and the area where the predator foraged. Stable isotope values of prey vary with its trophic position and geographic region. Nitrogen stable isotope values (δ15N) increase with trophic position, while carbon stable isotope values (δ13C) depend more on the carbon uptake by the primary producer and thus differs among habitats (Peterson and Frey 1987; Frey 1988; Hobson 1999; Cherel and Hobson 2007).
We aimed to describe the annual cycle of red-throated divers captured in the eastern German Bight. We focussed on migratory connectivity, how the breeding location influences the temporal pattern of annual movements and on individual site fidelity between years. In particular, we aimed to test the following hypotheses: (1) in accordance with ring recoveries of individuals from Sweden, Britain and other regions in the capture area (Okill 1994; Hemmingson and Eriksson 2002) red-throated divers display a low degree of migratory connectivity, in particular: (1a) individuals from one or more breeding region mix in one non-breeding area (capture site) and (1b) individuals from one breeding region spread during migration and their stationary non-breeding home ranges do not overlap, (2) the location of the breeding area (longitude/latitude) affects the timing and pattern of annual movements, (3) similar to the high site fidelity to their breeding areas (Okill 1992), individual red-throated divers repeatedly utilise the same areas during their key life history stages between years.
Methods
Fieldwork
We obtained positions of 33 red-throated divers equipped with Argos satellite transmitters (platform transmitter terminals, PTTs) in late winter to early spring (February–April) of 2015 to 2017. Birds were captured in the eastern part of the German Bight (North Sea), approximately 20 km west of the islands of Sylt and Amrum. We captured divers using the night-lighting technique (Whitworth et al. 1997; Ronconi et al. 2010). For a detailed description of tagging, see Burger et al. (2019), Kleinschmidt et al. (2019), Heinänen et al. (2020) and www.divertracking.com. We used implantable PTTs manufactured by Telonics, Inc. (40 units) and Sirtrack, Ltd (5 units). Transmitters were programmed using varying duty cycles with 3 or 4 transmission hours and 12–24 h intervals during winter and 60–68 h intervals between transmissions during the breeding season. Blood samples were taken and stored on Whatman FTA cards (Whatman FTA card technology, Sigma Aldrich) to sex the birds genetically. As the proportion of male birds (6 out of 33) was clearly underrepresented, we did not pursue further analyses regarding sex-specific differences, but combined male and female data for further analyses. A detailed description on the genetic sex determination is provided in the supplementary material (A9).
Data filtering
The tracking data were filtered to reduce noise from location fixes with low or unknown accuracy. Filtering followed the approach in Dorsch et al. (2019), Burger et al. (2019), and Heinänen et al. (2020) and was conducted using the package ‘argosfilter’ (Freitas et al. 2008) in R (R Core Team 2018). First, the sdafilter Filter (lat, lon, dtime, lc, vmax = 20) was applied. Then, all locations with unrealistic swimming/flying speeds were removed, using the McConnell algorithm (McConell et al. 1992), unless the point was located at less than 5 km from the previous location. Second, ArcGISv.10.1 (ESRI 2012) was used to further inspect the filtered dataset and any remaining obvious outliers, such as unrealistic positions were removed from the dataset. Finally, positions recorded during the first 2 weeks after the transmitter implantation were excluded from the data set due to possible impacts of capture and surgery on the behaviour during recovery period.
Altogether we received 29,053 satellite transmitter positions. After filtering, 22,744 positions were left for further analyses. Considering the quality of the data, we found that 39.4% (n = 8,962) of the positions were categorised in location classes 3–1 and 60.6% (n = 13,782) of positions were assigned to location classes 0-B (Table A2, CLS 2013).
Data collection and definition of terms and seasons
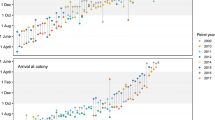
We used the migratory pattern observed in this dataset to define seasons within the annual cycle (Fig. 1, Table 1). The timing of site use varied from individual to individual and from year to year (Fig. 1). Therefore, we decided to define each season by the months in which at least one diver showed activity consistent with that season (i.e. migratory movements, or settlement during breeding, moulting or wintering season, Table 1). After spending some time on inland lakes, presumably for nesting, some red-throated divers moved to adjacent marine waters. Depending on the time period, they spent for nesting, these individuals are likely failed breeders or non-breeders. We did not consider these as staging periods as long as the diver stayed in the presumed breeding area.
Longitudinal migration pattern of all tracked red-throated divers (n = 33) during three consecutive years. Important regions utilised during breeding, moulting and wintering season are indicated on the right side. Individuals that did not show a clear breeding settlement are indicated with a dotted line
Autumn moult takes place in areas located along the migration route between breeding and wintering areas (Berndt and Drenckhahn 1990; Mendel et al. 2008) and is assumed to involve a stationary period of ≥ 21 days, including a flightless period. Hence, we divided the autumn migration from breeding to moult and from moult to wintering (Table 1).
We defined staging sites along migration routes as areas where an individual diver spent ≥ 5 days. Short stop-overs < 5 days were not considered in separate analyses. This classification of staging and stop-over behaviour followed Warnock (2010).
Analysing tracking data
PTTs were deployed over three separate years so ordinal date (day of the year) was used as the temporal variable allowing comparisons to be made across years.
We used ArcGISv.10.1 (ESRI 2012), QGISv2.18 (QGIS Development Team 2018) and two projections (Lambert Azimuthal Equal Area projection: ETRS89/ETRS-LAEA (EPSG: 3035), North Pole Azimuthal Equidistant projection (EPSG: 102016)) to inspect migratory patterns and to quantify migratory movements. We calculated migratory distances between capture and breeding sites by summing the length of all vectors created from point to point of the PTT locations from the first day of departure from a site to first day of arrival at the new site. We did not include movements within staging areas and sites into the distance calculation.
We used R (v 3.6.1) (R Core Team 2018) to analyse and plot longitudinal migratory patterns, home range estimates and repeatability of site use between 2 consecutive years. We used the R package stats4 (R Core Team 2018) to calculate correlations. A Pearson's correlation was run to determine the relationship between spatial (breeding longitude/latitude) and temporal variables (arrival/departure/time of stay). Additionally, we tested if the distance moved between capture area and breeding sites affected the duration of migration and staging behaviour.
For analysing migratory connectivity, we first defined the breeding regions to determine the extent to which individuals from a wintering area head to the same breeding region and use similar migratory routes. We divided breeding regions either by distance (> 700 km), or if separated by a large body of water (as breeding is constrained to land). Migratory routes were then assigned to the respective breeding region, namely Greenland, Scandinavia (Norway and Svalbard) and northern Russia. We analysed migratory connectivity between the breeding and non-breeding sites by quantifying the number of individuals of which positions were located along the same path using three analytical sections of the migration route (i) from the same starting point (capture site) to the same breeding region (n = 33), (ii) from one shared breeding region to their moulting destination (n = 19) and (iii) from one shared breeding region to their winter destination (n = 13). We analysed migratory connectivity during autumn migration (ii and iii) only for birds breeding in northern Russia (Siberian Arctic) as sample sizes from other regions were too small for further analyses. In addition, we assessed the strength of connectivity by calculating the Mantel correlation coefficient within the R package ade4 (Dray and Dufour 2007; Ambrosini et al. 2009; Trierweiler et al. 2014; Cohen et al. 2018). Statistical significance was determined using 9999 permutations (Trierweiler et al. 2014; Ambrosini et al. 2009). The Mantel correlation coefficient (rM) was calculated between pairwise (orthodromic) distance matrices of (i) individual positions at capture and breeding (n = 31 individuals with a fixed breeding position), (ii) breeding and moulting (n = 13 individuals breeding in Siberian Russia), and (iii) breeding and winter (n = 9 individuals breeding in Siberian Russia). Mantel correlation coefficient values range from 0 to 1, and indicate the strength of a population’s migratory connectivity. Values ≤ 0.25 suggest no spatial structure, values 0.26 to 0.50 indicate a weak structure, values 0.51 to 0.70 indicate a reasonable structure, and values > 0.71 indicate strong structure (Ambrosini et al. 2009).
We compared home range estimates for an overlapping site use during moult and winter (after the first breeding season) and between individuals from shared and non-shared breeding regions within the same season. We calculated 95% and 50% kernel density contours using the adehabitatHR package (Calenge 2011) in R, using h = ”LSCV/h-ref” as the smoothing parameter. We used only data from individuals that covered a full season and only one position of the best location class per day to avoid overrepresentation of some intervals. To calculate sizes of home ranges and core areas in km2, we converted the area of kernels to UTM units and created a new kernel (95% and 50%) based on the UTM coordinates. The UTM zone was chosen individually depending in which zone the estimated area was located using the WGS1984 datum.
We furthermore used these kernel density estimates to compare consistency in site use and a potential spatial overlap of individual home ranges between 2 consecutive years. When the same time period during winter (n = 4) and moult (n = 1) in consecutive years was available, 50% and 95% density contours were calculated. Consistency and flexibility of individual migratory movements, phenology and site utilisation between the 2 years was calculated using an ANOVA-based repeatability index (also called the intraclass correlation coefficient R) as an agreement of measurements between consecutive years (Nakagawa and Schielzeth 2010). The repeatability index offers information about the proportion of the total variation that is reproducible among repeated measurements of the same subject or group (Lessells and Boag 1987). The repeatability index is based on variance components derived from a one-way analysis of variance (ANOVA). This ANOVA-based method is one of the most commonly used methods to calculate repeatability in behavioural and evolutionary biology (Nakagawa and Schielzeth 2010) and has been applied in several studies on shore- and seabirds (Battley 2006; Vardanis et al. 2011; Conklin et al. 2013; Ruthrauff et al. 2019). The F table of an ANOVA, with the individual identities treated as factorial predictors, were used to calculate ANOVA-based repeatability estimates (RA). The repeatability (RA) was calculated by the formula introduced by Lessels and Boag (1987), where the mean between individual sum of squares (MSA), the mean within-individual (residual) sum of squares (MSw) and the sample size for each individual (2 years’ data) are considered. We considered all repeatabilities with 0 as no repeatability, all repeatabilites = 1 as total repeatability, all repeatabilities < 0.5 as low repeatability and all relatabilities > 0.5 as medium–high repeatability. The applicability of the method was confirmed in comparison with Linear mixed-effects model (LMM)-based methods (Stoffel et al. 2017), as both methods showed identical results (Appendix A3, Table A1). The utilisation of sites between 2 consecutive years was compared during spring staging (n = 9 individuals and 13 locations utilised in both years), breeding (n = 7 individuals) and moulting (n = 3 individuals by tracking data, n = 19 individuals by isotope data). We estimated repeatability between 2 consecutive years using Gaussian distributed data of position information, such as longitude/latitude on a small scale or isotope value on a broad scale (only for moult location) and the phenology (arrival/departure) using ordinal dates as the response variable and ID as the explanatory variable of the ANOVA (see Table 1, A1). Additionally, individual tracks were mapped where two seasons of each spring (n = 7) and autumn migration (n = 3) were available.
All estimates of averages are provided with standard deviations.
Stable isotope analyses
Stable isotope ratios are used in studies of the foraging ecology of seabirds, because they are proxies for the origin of resources (stable carbon isotope ratios δ13C) and relative trophic levels (stable nitrogen isotope ratios δ15N, e.g. Bedolla-Guzman et al. 2021). Feathers are used for stable isotope analyses, because feather proteins, formed during moult, reflect the stable isotope values of the diet at the time of their synthesis and can thus provide information on distribution and diet at the time of moult (Hobson and Clark 1992; Oppel and Powell 2008). Once grown, feathers are metabolically inert (Hobson 1999; Atkinson et al. 2005) and if potential moulting areas differ in their stable isotope values, it is possible to infer from δ13C values where the feather was grown (Hobson 1999).
We sampled the white neck feathers that are characteristic for the winter plumage and are grown during the autumn moult area (Streseman and Streseman 1966, Berndt and Drenckhahn 1974, Mendel et al. 2008) from all red-throated divers tracked during this project (n = 33). We used these feathers to determine the area where these feathers are grown and thus, the autumn moulting sites in the season previous to capture on a broad scale using stable isotope analyses. The white neck feathers of red-throated divers are particularly suitable for this purpose as they are easy to distinguish to ensure that this feather sample and its stable isotope values are representative of the autumn moult.
We linked isotope values to moulting regions for birds tracked with satellite transmitters, assuming that birds are faithful to regions between years. We used a sub-sample set of individual data (n = 10) provided by the satellite transmitters and determined the moult location of each bird to relate the isotopic information of feathers to geographic regions (North Sea vs. Baltic Sea). Then, we used these values to assign moult locations for the remaining birds tracked with satellite transmitters (n = 9) and for birds where no information from tracking data was available (n = 14). The feathers we sampled from satellite tracked birds were grown in the year before and thus tracking data collected in this study did not include the time when the sampled feathers were grown. Therefore, additionally literature of isotope values in the North and Baltic Sea were incorporated to confirm the classification. We revised carbon isotope values from muscle, eggshells and feathers of piscivorous vertebrates whose diets overlap with that of the red-throated divers (Das et al 2004, Céline Mahfouz et al. 2017, Corman et al 2018, Kleinschmidt et al. 2019, St John Glew et al. 2019, Christie 2021, Table 2) to obtain information on differences in carbon stable isotope values between the two seas. Stable isotope values were finally compared (North Sea vs. Baltic Sea) using the Wilks’ Lambda test and a One-way MANOVA (Bartlett Chi2) and the package rrcov (Todorov and Filzmoser 2009) to reveal if they statistically differ.
The isotope data were used to assign pre-capture moult locations to all birds tracked in this study (n = 33). When information about utilised moulting sites after capture was given by the tracking data (n = 19), it could be compared with the moulting sites assigned by isotope data. Therefore, moulting areas could be identified on a broad scale throughout the year and between years.
Samples were analysed at LIENSs Stable Isotope Facility at the University of La Rochelle. The treatment of feather samples and information about running the stable isotope analyses followed the approach described in Dorsch et al. (2019) and is provided in the supplementary material (A8).
Results
Migratory routes and utilised sites
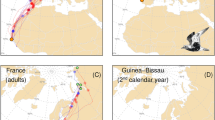
Breeding destinations of red-throated divers captured in the eastern German Bight (n = 33) covered the whole breeding range of the NW European wintering population (65°W–98°E) specified by Wetlands International (2018, 2022). The breeding areas included destinations in Greenland (n = 2), Svalbard (n = 2), Norway (n = 4) and northern Russia (n = 25) (Fig. 2). Divers from one capture site displayed both, a longitudinal migration (eastern direction to Russia, n = 25; western direction to Greenland, n = 2) and a latitudinal migration (central direction to Norway, n = 4, Svalbard, n = 2). Consequently, migratory directions are in the following termed as the Greenland direction, the Scandinavian direction (Svalbard and Norway) and the Russian direction. Of birds with a settlement in a breeding site in northern Russia (n = 24), 79% of breeding positions (n = 19) were located in the Siberian Arctic (Yamal, Gydan and Taimyr peninsulas and West Siberian Plain) (65°E–98°E) and 21% (n = 5) in the European part of northern Russia (Kola Peninsula, Kanin Peninsula, Pechora Sea (Tobseda Island) and Novaya Zemlya) (40°E–55°E). The migratory pathways of single individuals from all breeding areas were mixed on the route towards the Scandinavian direction (east Greenland n = 1, Svalbard n = 2, Norway n = 4, and northern Russia n = 1) and overlapped spatially from 54°N (capture site) till 68/70°N before leading to the final migration direction (Fig. 2).
1st year spring and autumn migration tracks of red-throated divers (n = 31, n = 19) from the capture location in the German Bight to their breeding areas (above) and from potential breeding locations to potential moulting locations and to wintering sites (below). Colours of migration tracks indicate different breeding regions (violet to Greenland; light blue to Svalbard; orange to Norway, black to the eastern arctic of northern Russia; grey to the Siberian arctic of northern Russia). Individual time of stay in spring staging areas along the migration route to Northern Russia for 32 staging stops performed by n = 23 individuals is visualized as zoom included in the map
Along the route to northern Russia (n = 25), we identified 12 staging sites of which 7 were located within the Baltic Sea. High frequented staging sites were the Skagerrak-Kattegat (7%), the Pomeranian Bight (10%), the Gulf of Bothnia and in particular the Gulf of Riga (24%, n = 9, Fig. 1, A4). In this context, the following winter showed that the capture area itself (eastern German Bight) served as a staging area (40%, n = 10) if individuals spent the winter elsewhere (Fig. 4).
In autumn, on the way back from the breeding areas, most red-throated divers followed the directions and pathways they have already used during the spring migration with 77% of red-throated divers performing a step-wise migration with separate moult and winter migrations (Figs. 2 and 6).
All birds moulting in the North Sea according to tracking data had δ13C > -18‰, while birds that moulted in the Baltic Sea had δ13C < -18‰. This threshold was confirmed by the literature values. We thus applied this classification to all birds tracked through the annual cycle (n = 33) and assigned moulting regions in the Baltic Sea and the North Sea to a similar number (51% and 49%, respectively). The isotope values from red-throated divers probably indicated a split between moulting in North Sea and Baltic Sea (Fig. 3). Clusters of SI were significantly different between samples (Wilks' Lambda = 0.084, χ2 = 76.61, DF = 2.00, p < 0.001) that would, based on literature on other species (Table 2), be considered to come from the Baltic Sea or from the North Sea.
Tracking data revealed that wintering sites were distributed in the Baltic Sea, North Sea (eastern German Bight and southern Bight) and Irish Sea with the highest proportion using the eastern German Bight (60%, n = 10, Fig. 4) either during the complete season or temporarily.
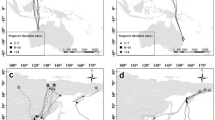
Areas used by red-throated divers during moult (above) and during winter (below). The legend on top informs about individual colours and corresponding breeding region. Estimated Kernel densities 95% and 50% for utilised areas in Baltic and North Sea during moult season (n = 13) and in Baltic, North and Irish Sea during winter season (n = 10) for a time period that lasts from the first date in the area (arrival) until last date (departure). Individual maps are displayed when birds utilised the same area and their home ranges are not distinguishable, (moult: Eastern German Bight n = 2, Bay of Riga n = 6, winter: Eastern German Bight n = 5). 95% kernel density contours are displayed with 70% transparency and 50% kernel density (core habitat use) with 50% transparency
Migratory connectivity
Starting on the capture site, red-throated divers spread out over a large geographic range for breeding (Figs. 2 and 5). Considering connectivity among individuals from one breeding region, only individuals from northern Russia were included as the sample size for the other regions were too small to show meaningful results. Individuals breeding in northern Russia used mainly routes via the Baltic Sea to migrate to and from breeding sites, with one exception that moved along the Northern Cape (Fig. 2). During spring migration individuals breeding in northern Russia showed no consistent pattern with varying staging stop locations, staging stop durations and travel times (Fig. 2, Table 3). These individuals spread out to several moulting sites (stationary period from September to December > 21 days) in the North and Baltic (38% and 62%, respectively, Figs. 2, 4 and Fig. 5). Within the Baltic Sea, the majority of birds (n = 67%) spent the potential moulting time in the Gulf of Riga. During winter, 50% of the tracked birds breeding in northern Russia utilised the German Bight, whereas the other 50% distributed elsewhere (25% in the southern Bight, 8% in the Irish Sea and 17% in the Baltic Sea), (Figs. 4 and 5). Kernel density estimation of individuals from one breeding region showed only partly overlapping home ranges but these individuals mixed with individuals from other breeding regions (northern Russia n = 4, Svalbard n = 1, Fig. 4). The distance between individual areas within the moulting period as well as within the winter period was up to 1000 km. Combination of tracking data (n = 19, Figs. 4 and 5) and additional birds determined by stable isotopes (n = 13) revealed that individuals that moulted in the Baltic Sea (n = 15) migrated all from northern Russia but red-throated divers that moulted in the North Sea (n = 17) were composed of individuals from several breeding regions, northern Russia (52.9%), Norway (23.5%), Svalbard (11.8%) and Greenland (11.8%). Migratory patterns varied between individuals with the majority performing a separate moult and autumn migration and a few individuals performing a direct migration (migration to a site that was utilised during moult and winter).
Population spread and interpopulation mixing of red-throated divers from the NW European winter population starting at the capture site (eastern German Bight) heading to breeding regions and from breeding regions to moult and winter sites. From capture site to breeding data are based on tracking data, from breeding to moult data are combined of tracking and additional birds determined by stable isotopes and from moult to winter data are based on tracking data. Each breeding region is presented in a specific colour consistent with the with the division made in Fig. 1 (violet = Greenland, orange = Norway, light blue = Svalbard and dark blue = Northern Russia) and Boxes show number of individuals using this region
Quantification of migratory connectivity between individuals captured in the German Bight (eastern North Sea) indicated no relation between individuals from one breeding region (i) to moulting or (ii) wintering sites. Calculation of a Mantel correlation coefficient indicate no spatial structure and a low connectivity but gave no significant results: capture site to breeding: rM; = 0.069, n = 31, p = 0.202; breeding to moult (Siberian ind.): rM = 0.135, n = 13, p = 0.215; breeding to winter (Siberian ind.): rM = 0.274, n = 9, p = 0.103.
Thus, individuals from northern Russia spread out to several winter sites with no uniform pattern of individuals from this breeding region and various utilisation areas and mix with individuals from other breeding regions (Figs. 4 and 5).
Timing of migration and geographic relations
Migration distances can only be given as minimum estimates assuming straight flight paths between consecutive Argos positions. Referring to Cox (2010) and Rappole (2013), the majority of red-throated divers in this study migrated > 1000 km and can be considered as long-distance migrants (87.9%) and just a small number of birds moved short distances < 1000 km to Norway (12.1%) (Table 3).
Breeding location was significantly correlated with duration of migration (longitude: r = 0.407, n = 29, p = 0.027; latitude: r = 0.384, n = 29, p = 0.039). Overall, individuals breeding at a higher longitude in Russia needed a longer travelling time (Fig. A2a). The longest travel time during spring migration was 65 days and this bird headed to Taymir Peninsula in northern Russia. Departure date (ordinal date-day of year) from wintering sites was significantly correlated with breeding latitude but not with breeding longitude (longitude: r = 0.335, n = 9, p = 0.344; latitude: r = 0.714, n = 9, p = 0.020). More northerly located breeders departed later from their wintering site than southerly located breeders, suggesting a latitudinal gradient (Fig. A2c). Arrival date to breeding areas (ordinal date-day of year) was significantly correlated with a higher breeding longitude but not with a higher breeding latitude (longitude: r = 0.695, n = 29, p < 0.001; latitude: r = 0.373, n = 29, p = 0.078). More easterly located breeders arrived later at their breeding sites than westerly located breeders with up to 40 days later arrival, suggesting a longitudinal gradient (Fig. A2b). Departure date from breeding sites was neither correlated with breeding longitude (n = 20, p = 0.941) nor with breeding latitude (n = 20, p = 0.285, Fig. A1b).
We observed no correlation between breeding positions (long/lat) and duration of staging (n = 25, p = 0.852, Fig. A1a) but a significant correlation between a longer travelling time and a longer duration of staging (r = 0.643, n = 27, p < 0.001, Fig. A3) that was correlated with a higher number of staging stops (rs = 0.468, n = 27, p = 0.014, Fig. A3). Although staging time was positively correlated with travelling time, the distance itself had no effect on either staging time (n = 25, p = 0.986, Fig. A1c) or travelling time (n = 31, p = 0.116, Fig. A1c).
Repeatability of year-round movements and site utilisation between consecutive years
Not all birds caught in the German Bight in winter and spring returned to this location during the following winter. 32% of the tracked birds used this area for moult and 54% for wintering. Individuals that did not use this area during moult or wintering used this area as a staging site or a short stop along migration.
The temporal pattern and repeated site utilisation with regard to spring staging sites, breeding and moulting areas showed high individual consistency between years (Fig. 6, Table A1). Visual inspection of individual migratory pathways between consecutive years showed similar movements in six of seven individuals, however, one individual (146,444) used different pathways between two spring migrations (Fig. 6). Kernel density estimates from individuals for which tracking data were available from an overlapping period during moult and winter in consecutive years showed that home ranges overlapped or were close, indicating consistent site use in consecutive years (Fig. 6).
Migration routes of individuals during two subsequent years (first year = solid line, second year = dotted line, the black arrow indicates the direction of movement). The left side shows individual spring migration tracks marked by colour (n = 7) from capture site to potential breeding sites in the first year and from wintering site to potential breeding sites in the second year. The right side shows individual moult migration tracks marked by colour from potential breeding sites to potential moulting sites (n = 3) and to wintering sites (n = 1) for two consecutive seasons. Individual home ranges when data transmission allowed for two overlapping time periods during winter and moult in consecutive years are visualized as zoom included in the map
Repeatability (Table A1) towards number of individual spring staging stops was moderate between years (RA = 0.407, F8,9 = 2, p = 0.161). The repeatability towards individual spring staging longitudes (RA = 0.954, F 12,13 = 42.9, p < 0.001) and spring staging latitudes (RA = 0.982, F12,13 = 107.8, p < 0.001), that appeared in both years, was high. High repeatability’s were also found for breeding longitudes (RA = 1, F6,7 = 24,945, p < 0.001), breeding latitudes (RA = 1, F6,7 = 9125, p < 0.001) and moulting locations by isotope analyses (RA = 0.785, F18,19 = 8.278, p < 0.001). Between year repeatability of migratory timing indicated a less consistent pattern for arrival times in spring staging times (RA = 0.604, F12,13 = 4.1, p = 0.009), departure times from spring staging sites (RA = 0.751, F12,13 = 7.0, p = 0.001), arrival times in breeding areas (RA = 0.552, F6,7 = 3.5, p = 0.064), departure times from breeding areas (RA = 0.408, F3,4 = 2.4, p = 0.211) and arrival times in moulting areas (RA = 0.876, F2,3 = 15.11, p = 0.027).
Discussion
Based on tracking data and stable isotope analyses, we obtained a comprehensive dataset on annual movements of NW European red-throated divers that addressed our hypotheses as outlined below. Tracking data lasted for up to 2 consecutive years and thus allowed to assign individual site utilisation within and between years. Stable isotope analyses added information about moulting sites where no tracking data were available. Isotopic values in our study seem to be clearly separable between North and Baltic Sea and in line with the locations determined by the tracking data. Although matching of isotope data and tracking data without temporal overlap may have some uncertainties, published isotopic values from the North and Baltic Sea backed up our assignment of moulting sites. This approach has previously been used successfully by Oppel and Powell (2008) to determine winter locations of eiders (Somateria spectabilis). They also used information from satellite tracked birds to isotopically delineate regions and assigned feathers of birds not tracked with satellite transmitters to regions using their stable isotope values.
Do red-throated divers have a low degree of migratory connectivity?
Considering ring recoveries in coastal areas around the North Sea (Okill 1994), we expected a mix of individuals from several breeding regions in this area and thus a low degree of connectivity. We found individuals from four different breeding regions captured in one local area during late winter and early spring. The distances and water bodies between these breeding regions indicates a delimitation of these regions. We captured red-throated divers in one relatively local wintering/spring staging site and not in the breeding area, as most other studies analysing migratory connectivity do. A possible bias could have been that the connectivity would have been overestimated if all birds would have headed from the same winter region to the same breeding region and back. In this case, individuals from a shared breeding region that use other winter regions, would have been missed as they were out of our sample range. In our case, however, we found a relatively high spread from individuals heading to distant breeding regions and of individuals from one breeding region to several non-breeding regions and therefore this bias is unlikely to affect the results. However, that individuals migrated along their routes to a shared breeding area in northern Russia indicates some degree of connectivity, as most of these individuals moved along the Baltic Sea and used similar staging sites along this route. In another study, McCloskey et al. (2018) tagged red-throated divers in four breeding regions in Alaska. These individuals also followed similar migration routes, indicating some degree of connectivity, but did not display a discrete use of population-specific non-breeding areas. The fact that almost all red-throated divers in this study that bred in northern Russia migrated along the Baltic Sea could also be due to the fact, that they follow an established migration route that is used by various species of waterfowl, the Northeast Atlantic Flyway (BirdLife International 2010), rather than to the fact that they exhibit community-specific patterns (Boere and Straud 2006). On a smaller scale and considering site utilisation during the stationary non-breeding season (moult and winter), red-throated divers in this study spread to distinct areas. Following the ‘weak–strong’ continuum defined by Webster et al. (2002), this pattern would suggest that red-throated divers displayed a low or diffuse connectivity with individually variable movements and no specific or uniform pattern of individuals from one site or migratory direction. Statistical tests showed no significant correlation between utilised breeding regions, moulting or wintering sites, which supports the low connectivity indicated by Fig. 5. Also, kernel density estimation during moult and winter in the season after capture showed only partly overlapping home ranges between individuals from a given breeding region (Fig. 4). Our results are consistent with the study of Gray (2021) who found low connectivity of red-throated divers in eastern North America, indicating that red-throated divers display a highly individual movement behaviour that is adapted rather to individual qualities and environmental conditions than to community-specific patterns.
Does the location of breeding area (longitude/latitude) affect the timing and spatial pattern of annual movements?
Like other species, red-throated divers seem to follow an endogenous schedule of migration together with the strong phenological gradient along the spring migration route to Arctic breeding areas (Gordo 2007; McNamara et al. 2011; Shariatinajafabadi et al. 2014; Smith et al. 2020). Arrival dates in more westerly located Arctic breeding sites were about 40 days earlier than arrival in more easterly Arctic breeding sites, indicating a longitudinal gradient. We did not find a correlation between departure from breeding sites and breeding location, which could be related to variations in breeding success. Birds from more northern breeding areas departed later from wintering areas, consistent with the pattern of later arrival at breeding areas. The temporal pattern seems to confirm that timing of migration appears to follow environmental conditions (e.g. growing seasons, ice-free conditions and temperatures) in arctic breeding regions, similar to other waterfowl and shorebirds (Schwartz 1998; Shariatinajafabadi et al. 2014; Smith et al. 2020). Migratory movements and breeding events of shorebirds and avian herbivores can be constrained by plant phenology and spring salt marsh productivity (Shariatinajafabadi et al. 2014; Smith et al. 2020). Winkler et al (2014) stated that migration strategies can be seen as the mapping of actions (e.g. feeding, departure) on cues (e.g. daylength, feeding or wind conditions). Although red-throated divers are piscivorous seabirds and do not directly depend on plant phenology, other factors such as seasonal day length and temperatures could be indicators that lead birds to hit the right time with suitable conditions at breeding sites. In this case, a later arrival time at more easterly located breeding locations might also explain why a longer travel time (duration of the spring migration) was significantly correlated with a more easterly breeding location, but not with distance travelled (Fig. A1c, A2a). Another factor that should be taken into account here is the individual need to refuel along the route (Weber and Houston 1997). We found a significant correlation between a longer travelling time and a longer duration of staging and a higher number of staging stops (Fig. A30). In this context, as medium-sized birds with weight varying between 1400 and 2000 g (own observations) and high wing loading (Storer 1958; Lovvorn and Jones 1994) using flapping flight, the energy expenditure of divers is relatively high (Pennycuick 1989). To refuel energy reserves, divers, travelling to more distant areas (longitudes) may, therefore, need more and longer staging stops, thus increasing the total travel time. The high energy expenditure though might be counteracted by the use of favourable wind conditions (tailwinds), or very good foraging conditions at staging sites. However, our results are in line with the finding of McCloskey et al. (2018) and Gray (2021) who found red-throated divers to perform long/slow migrations with many stop-overs.
Do individuals faithfully utilise areas during their key life history stages between years?
Information about individual consistency between years helps to understand the capacity to cope with habitat change and selection pressures (Dias et al 2010, Conklin et al. 2013, McFarlane et al 2014, Merkel et al. 2020). Based on a high site fidelity observed in breeding areas (Okill 1992; Poessel et al. 2020), we expected a similar high site fidelity for the non-breeding sites. The sample size for individual site utilisation in two consecutive years was rather small with data of (n = 9) individuals for spring migration and of (n = 7) individuals for breeding sites. The data set of (n = 3) individuals tracked for moulting sites could be enhanced by the isotopic data (n = 19). Although the sample size is rather small, the results of the repeatability analyses of annual migratory movements seem to confirm and extend previous studies on site fidelity (Okill 1992; Poessel et al. 2020).
Similar to the strong winter site fidelity (85%) of common loons/ great-northern divers (Gavia immer) shown by Paruk et al. (2015) red-throated divers exhibit a relatively high fidelity towards the different areas visited during the annual cycle: migration routes, staging, breeding and moulting areas, however, with some variation for individuals and temporal pattern. Home ranges estimated by Kernel densities in 2 consecutive years and during the same time period in moult and winter could only be shown on the basis of a few individuals (n = 4), but revealed that these individuals had either some home range overlap in consecutive seasons, or the home ranges were located in the same area and close to each other. Home range estimation and calculation of repeatability might indicate that site selection is driven by macro-selection of a larger area with sufficient frequency of opportunistic prey encounters. Once such a profitable area with suitable feeding conditions is found, it is used faithfully from year to year. In this study, we only have 2 years of data and in the context of fidelity and flexibility, it is equally plausible that red-throated divers exhibit fidelity only until a site of use is no longer suitable, in which case the flexibility of the divers would be expressed. Skov and Prins (2001) have shown that the eastern German Bight is known to be particularly attractive to red-throated divers due to the frontal zone and resulting favourable feeding conditions with suitable prey species (Guse et al. 2009; Kleinschmidt et al. 2019). If habitat selection is driven by a macro-selection, it could be that habitat change has an effect on a smaller spatial scale and displacement is a process happening at smaller scales. However, in this context, results from studies on red-throated diver distributions and displacement effects in the eastern German Bight showed that red-throated divers remained in the general area, but shifted their distribution and congregated outside disturbed areas (Mendel et al. 2019; Vilela et al. 2021). Mendel et al. (2019) analysed long-term datasets of aerial and ship based surveys, whereas Vilela et al. (2021) analysed long-term datasets of aerial surveys. However, no population decline was observed (Vilela et al. 2021). Considering this, our data based on the visualised tracks, single home ranges (Fig. 6) and statistical analyses of individual repeatability (Table A1) show an individual consistent site use in consecutive years, considered over a broad scale. Combined with the findings of Mendel et al. (2019) and Vilela et al. (2021), these data suggest that red-throated divers are somewhat flexible to change sites at small scales, but may have limited flexibility to change sites at large scales in response to large-scale habitat loss.
Arrival and departure times were more consistent at non-breeding sites than at breeding sites. The high consistency in arrival times in moulting areas might limit their flexibility in responding to anthropogenic change during that time period when birds are flightless. Similar to other medium-to-large sized diving bird species, divers are expected to perform a synchronous wing moult (Thompson and Kitaysky 2004) rendering them flightless and thus require undisturbed areas during this time. Arctic breeding areas on the other hand are characterised by a short arctic summer and thus a narrow seasonal window where breeding can take place (Klaassen 2003). Therefore, this Arctic breeding bird species may have adapted to a more flexible timing of arrival to match optimal conditions in breeding areas, triggered by colder or warmer winter or spring temperatures.
Conclusions and implication for conservation
In agreement with prior research (e.g., Mendel and Garthe 2010; Dierschke et al. 2012; Mendel et al. 2019), our study confirms the importance of the North Sea, in particular the eastern German Bight, as a wintering area, staging site before spring migration and moulting area for red-throated divers. The consistent use of the Gulf of Riga in our study in spring and autumn confirmed that this area is another important site for red-throated divers migrating from northern Russia and moving to the North Sea and adjacent waters (Berndt and Drenckhahn 1990; Helcom 2013).
Low connectivity might indicate resilience to environmental change on a population level, but the high fidelity towards sites during the stationary non-breeding season indicates a rather high consistency of annual movements which may result in a low individual flexibility. These findings are highly important to be considered for future appropriate conservation measures. All divers in this study were captured in the eastern German Bight but migrated to separate breeding areas and used this area with varying intensity in the following season. The observed low or diffuse connectivity of individuals from one breeding region distributes the effect to only a proportion of individuals from each breeding region. Compared to a high connectivity, where all individuals from one breeding region would experience the same non-breeding conditions over the same time in this area, a higher resilience can be suggested (Newton 2008; Rushing et al. 2016). Interannual movements of red-throated divers on the other hand showed a relatively high individual repeatability and consistent site use. Consistent use of high energetic mobile prey species (Guse et al. 2009; Kleinschmidt et al. 2019) indicates that the occurrence of these prey species seem to be an important habitat criteria. Considering the use of multiple core areas during winter and the dependence of divers on these mobile prey species in dynamic marine habitats, could also indicate some flexibility. Regarding anthropogenic pressures and altered environmental conditions, a poor wintering habitat quality can carry-over to breeding sites and influence reproductive success (Marra et al. 1998; Moore 2005; Harrison et al. 2011; Rushing et al. 2016). The winter population of red-throated divers shows strong avoidance towards the increasing anthropogenic pressure (Garthe et al. 2015; Mendel et al. 2019) but does not decline (Vilela et al. 2021). The low connectivity could counteract a quick population decline by having only small effects on populations of this long-lived species. If the impact always affects only one number or a proportion, but not the entire population, it may take longer for the impact to become apparent. More research on reproductive success in the arctic breeding regions is needed to link population estimates between breeding and non-breeding areas. If a breeding population experiences individually different travel times, caused by altered conditions in the non-breeding areas, this may result in different arrival times at the breeding site and a possible mismatch (Marra et al. 1998; Moore 2005; Rushing et al. 2016). It should also be noted here that, climate warming can alter ice-free periods in Arctic breeding areas which has the potential to alter the timing of migration (Walther et al. 2002; Catry et al. 2013; Wauchope et al. 2017).
Although anthropogenic pressures in the North Sea appear to be distributed among individuals from multiple populations, when considered cumulatively and taking into account individuals breeding in northern Russia, multiple threats during migration come together, such as gillnet fishing and pollution of the Baltic Sea (Dagys and Žydelis 2002; Rubarth et al. 2011; Žydelis 2013). When it comes to future spatial planning, our data support the finding that all information on species abundance and sites used along the migration route needs to be considered, regardless of whether they are geographically or politically distant (Runge et al. 2014; Johnston et al. 2020).
Data availability
All data have been deposited in the Movebank repository.
References
Alves JA, Gunnarsson TG, Hayhow DB, Appleton GF, Potts PM, Sutherland WJ, Gill JA (2013) Costs, benefits, and fitness consequences of different migratory strategies. Ecology 94(1):11–17
Ambrosini R, Møller AP, Saino N (2009) A quantitative measure of migratory connectivity. J Theor Biol 257(2):203–211
Atkinson PW, Baker AJ, Bevan RM, Clark NA, Cole KB, Gonzalez PM, Newton J, Niles LJ, Robinson RA (2005) Unravelling the migration and moult strategies of a long-distance migrant using stable isotopes: red knot Calidris canutus movements in the Americas. Ibis 147(4):738–749
Battley P (2006) Consistent annual schedules in a migratory shorebird. Biol Let 2(4):517–520
Bedolla-Guzmán Y, Masello JF, Aguirre-Muñoz A, Lavaniegos BE, Voigt CC, Gómez-Gutiérrez J, Sánchez-Velasco L, Robinson CJ, Quillfeldt P (2021) Year-round niche segregation of three sympatric hydrobates storm-petrels from Baja California Peninsula, Mexico, Eastern Pacific. Mar Ecol Prog Ser 664:207–225
Berndt RK, Drenckhahn D (1990) Vogelwelt Schleswig-Holsteins 1: Seetaucher bis Flamingo. (2. korr. Aufl. Auflage). Wachholtz/Neumünster, pp 27–37
BirdLife International (2010) The flyways concept can help coordinate global efforts to conserve migratory birds. http://datazone.birdlife.org/sowb/casestudy/the-flyways-concept-can-help-coordinate-global-efforts-to-conserve-migratory-birds. Accessed 01 Mar 2022
BirdLife International (2022) Species factsheet: Gavia stellata. . http://www.birdlife.org. Accessed 12 June 2022
Boere GC, Stroud DA (2006) The flyway concept: what it is and what it isn’t. In: Boere GC, Galbraith CA, Stroud DA (eds) Waterbirds around the world. The Stationery Office, Edinburgh, UK, pp 40–47
Burger C, Schubert A, Heinänen S, Dorsch M, Kleinschmidt B, Žydelis R, Morkūnas J, Quillfeldtdt P, Nehls G (2019) A novel approach for assessing effects of ship traffic on distributions and movements of seabirds. J Environ Manag 251:109511
Calenge C (2011) Home range estimation in R the adehabitatHR package. Office national de la classe et de la faune sauvage: Saint Benoist, Auffargis, France
Catry P, Encarnação V, Araújo A, Fearon P, Fearon A, Armelin M, Delaloye P (2004) Are long-distance migrant passerines faithful to their stopover sites? J Avian Biol 35(170):181
Catry P, Dias MP, Phillips RA, Granadeiro JP (2013) Carry-over effects from breeding modulate the annual cycle of a long-distance migrant: an experimental demonstration. Ecology 94(6):1230–1235
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329:281–287
Christie AP (2021) Investigating post-breeding moult locations and diets of common guillemots (Uria aalge) in the North Sea using stable isotope analyses. bioRxiv. https://doi.org/10.1101/2020.09.01.276857
CLS (2013) Argos user’s manual. http://www.argos-system.org/manual. Accessed 15 June 2016
Cohen EB, Hostetler JA, Hallworth MT, Rushing CS, Sillett TS, Marra PP (2018) Quantifying the strength of migratory connectivity. Methods Ecol Evol 9(3):513–524
Conklin JR, Battley PF, Potter MA, Fox JW (2010) Breeding latitude drives individual schedules in a trans-hemispheric migrant bird. Nat Commun 1(1):1–6
Conklin JR, Battley PF, Potter MA (2013) Absolute consistency: individual versus population variation in annual-cycle schedules of a long-distance migrant bird. PLoS ONE 8(1):e54535. https://doi.org/10.1371/journal.pone.0054535
Corman AM, Schwemmer P, Mercker M, Asmus H, Rüdel H, Klein R, Boner M, Hofem S, Koschorreck J, Garthe S (2018) Decreasing δ13C and δ15N values in four coastal species at different trophic levels indicate a fundamental food-web shift in the southern North and Baltic seas between 1988 and 2016. Environ Monit Assess 190(8):1–12
Cox GW (2010) Bird migration and global change. Island Press
Cramp S, Simmons KEL (1977) Birds of the western Palaearctic. Vol. I. Ostrich to ducks. Oxford University Press, Oxford
Cresswell W (2014) Migratory connectivity of Palaearctic-African migratory birds and their responses to environmental change: the serial residency hypothesis. Ibis 156:493–510
Croxall JP, Silk JRD, Phillips RA, Afanasyev V, Briggs DR (2005) Global circumnavigations: tracking year-round ranges of nonbreeding albatrosses. Science 307:249–250
Dagys M, Žydelis R (2002) Bird bycatch in fishing nets in Lithuanian coastal waters in wintering season 2001–2002. Acta Zool Litu 12(3):276–282
Das K, Siebert U, Fontaine M, Jauniaux T, Holsbeek L, Bouquegneau JM (2004) Ecological and pathological factors related to trace metal concentrations in harbour porpoises Phocoena phocoena from the North Sea and adjacent areas. Mar Ecol Prog Ser 281:283–295
Dias MP, Granadeiro JP, Phillips RA, Alonso H, Catry P (2010) Breaking the routine: individual Cory’s shearwaters shift winter destinations between hemispheres and across ocean basins. Proc R Soc B 278:1786–1793. https://doi.org/10.1098/rspb.2010.2114
Dierschke V, Exo KM, Mendel B, Garthe S (2012) Gefährdung von Sterntaucher Gavia stellata und Prachttaucher G. arctica in Brut-, Zug-und Überwinterungsgebieten–eine Übersicht mit Schwerpunkt auf den deutschen Meeresgebieten. Vogelwelt 133:163–194
Dorsch M, Burger C, Heinänen S, Kleinschmidt B, Morkūnas J, Nehls G, Quillfeldt P, Schubert A, Žydelis R (2019) DIVER – German tracking study of seabirds in areas of planned offshore wind farms at the example of divers. Final report on the joint project DIVER, FKZ 0325747A/B, funded by the federal ministry of economics and energy (BMWi) on the basis of a decision by the German Bundestag. https://bioconsult-sh.de/en/about-us/documents. Accessed 14 Mar 2022
Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22(4):1–20
Duijns S, Niles LJ, Dey A, Aubry Y, Friis C, Koch S, Anderson AM, Smith PA (2017) Body condition explains migratory performance of a long-distance migrant. Proc R Soc B. https://doi.org/10.1098/rspb.2017.1374
Environmental systems research institute (ESRI) (2012) ArcGIS release 10.1. Redlands, CA
Esler D (2000) Applying metapopulation theory to conservation of migratory birds. Conserv Biol 14:366–372
Fayet AL, Freeman R, Anker-Nilssen T, Diamond A, Erikstad KE, Fifield D, Kouwenberg AL, Kress S, Mowat S, Perrins CM, Petersen A, Petersen IK, Reiertsen TK, Robertson GJ, Shannon P, Sigurðsson IA, Shoji A, Wanless S, Guilford T (2017) Ocean-wide drivers of migration strategies and their influence on population breeding performance in a declining seabird. Curr Biol 27(24):3871–3878
Finch T, Butler SJ, Franco AM, Cresswell W (2017) Low migratory connectivity is common in long-distance migrant birds. J Anim Ecol 86(3):662–673
Freitas C, Lydersen C, Fedak MA, Kovacs KM (2008) A simple new algorithm to filter marine mammal Argos locations. Mar Mamm Sci 24(2):315–325
Frey B (1988) Food web structure on Georges Bank from stable C, N and S isotopic compositions. Limnol Oceanogr 33(5):1182–1190
Garthe S, Sonntag N, Schwemmer P, Dierschke V (2007) Estimation of seabird numbers in the German North Sea throughout the annual cycle and their biogeographic importance. Vogelwelt 128(4):163–178
Garthe S, Schwemmer H, Markones N, Müller S, Schwemmer P (2015) Distribution, seasonal dynamics and population trend of divers Gavia spec. in the German bight (North Sea). Vogelwarte 53:121–138
Gilroy JJ, Gill JA, Butchart SH, Jones VR, Franco AM (2016) Migratory diversity predicts population declines in birds. Ecol Lett 19(3):308–317
Gonzalez-Solıs J, Croxall JP, Oro D, Ruiz X (2007) Trans-equatorial migration and mixing in the wintering areas of a pelagic seabird. Front Ecol Environ 5:297–301
Gordo O (2007) Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Clim Res 35(1–2):37–58
Gray CE (2021) Migration and winter movement ecology of red-throated loons (Gavia stellata) in eastern North America. Dissertations, The University of Maine
Gunnarsson TG, Tómasson G (2011) Flexibility in spring arrival of migratory birds at northern latitudes under rapid temperature changes. Bird Study 58(1):1–12
Guse N, Garthe S, Schirmeister B (2009) Diet of red-throated divers Gavia stellata reflects the seasonal availability of Atlantic herring Clupea harengus in the southwestern Baltic Sea. J Sea Res 62(4):268–275
Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S (2011) Carry-over effects as drivers of fitness differences in animals. J Anim Ecol 80(1):4–18
Heinänen S, Žydelis R, Kleinschmidt B, Dorsch M, Burger C, Morkūnas J, Quillfeldtdt P, Nehls G (2020) Satellite telemetry and digital aerial surveys show strong displacement of red-throated divers (Gavia stellata) from offshore wind farms. Mar Environ Res. https://doi.org/10.1016/j.marenvres.2020.104989
HELCOM (2013) Species information sheet: Gavia stellata. http://www.helcom.fi/Red%20List%20Species%20Information%20Sheet/HELCOM%20Red%20List%20Gavia%20stellata%20(wintering%20population).pdf Accessed 4 Sept 2018
Hemmingsson E, Eriksson MO (2002) Ringing of red-throated diver Gavia stellata and black-throated diver Gavia arctica in Sweden. Wetl Int Diver/loon Spec Group Newsl 4:8–13
Hobson KA (1999) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120(3):314–326
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor 94:181–188
Johnston A, Auer T, Fink D, Strimas-Mackey M, Iliff M, Rosenberg KV, Brown S, Lanctot R, Rodewald AD, Kelling S (2020) Comparing abundance distributions and range maps in spatial conservation planning for migratory species. Ecol Appl. https://doi.org/10.1002/eap.2058
Klaassen M (2003) Relationships between migration and breeding strategies in arctic breeding birds. Avian migration. Springer, Berlin, Heidelberg, pp 237–249
Kleinschmidt B, Burger C, Dorsch M, Nehls G, Heinänen S, Morkūnas J, Žydelis R, Quillfeldt P (2019) The diet of red-throated divers (Gavia stellata) overwintering in the German bight (North Sea) analysed using molecular diagnostics. Mar Biol 166(6):1–18
Klinner T, Buddemeier J, Bairlein F, Schmaljohann H (2020) Decision-making in migratory birds at stopover: an interplay of energy stores and feeding conditions. Behav Ecol Sociobiol 74(1):1–14
Kokko H (1999) Competition for early arrival in migratory birds. J Anim Ecol 68:940–950
Kölzsch A, Müskens GJ, Kruckenberg H, Glazov P, Weinzierl R, Nolet BA, Wikelski M (2016) Towards a new understanding of migration timing: slower spring than autumn migration in geese reflects different decision rules for stopover use and departure. Oikos 125(10):1496–1507
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104(1):116–121
Lovvorn JR, Jones DR (1994) Biomechanical conflicts between adaptations for diving and aerial flight in estuarine birds. Estuaries 17(1):62
Mahfouz C, Meziane T, Henry F, Abi-Ghanem C, Spitz J, Jauniaux T, Bouveroux T, Gaby K, Amara R (2017) Multi-approach analysis to assess diet of harbour porpoises Phocoena phocoena in the southern North Sea. Mar Ecol Prog Ser 563:249–259
Marra PP, Hobson KA, Holmes RT (1998) Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282(5395):1884–1886
Marra P, Cohen E, Harrison AL, Studds C, Webster M (2018) Migratory connectivity. Conserv Biol Ser-Camb 14:157
Martin TG, Chadès I, Arcese P, Marra PP, Possingham PP, Norris DR (2007) Optimal conservation of migratory species. PLoS ONE 2:3–7
McCloskey SE, Uher-Koch BD, Schmutz JA, Fondell TF (2018) International migration patterns of red-throated loons (Gavia stellata) from four breeding populations in Alaska. PLoS ONE. https://doi.org/10.1371/journal.pone.0189954
McConnell BJ, Chambers C, Fedak MA (1992) Foraging ecology of southern elephant seals in relation to the bathymetry and productivity of the Southern Ocean. Antarct Sci 4(4):4393–4398
McFarlane T, Montevecchi LA, Fifield WA, Hedd DAA, Gaston AJ, Robertson GJ, Phillips RA (2014) Individual winter movement strategies in two species of murre (Uria spp.) in the Northwest Atlantic. PLoS ONE. https://doi.org/10.1371/journal.pone.0090583
McNamara JM, Barta Z, Klaassen M, Bauer S (2011) Cues and the optimal timing of activities under environmental changes. Ecol Lett 14(12):1183–1190
Mendel B, Garthe S (2010) Kumulative Auswirkungen von Offshore-Windkraftnutzung und Schiffsverkehr am Beispiel der Seetaucher in der Deutschen Bucht. Coastline Rep 15:31–44
Mendel B, Schwemmer P, Peschko V, Müller S, Schwemmer H, Mercker M, Garthe S (2019) Operational offshore wind farms and associated ship traffic cause profound changes in distribution patterns of loons (Gavia spp.). J Environ Manag 231:429–438
Mendel B, Sonntag N, Wahl J, Schwemmer P, Dries H, Guse N, Müller S, Garthe S (2008) Artensteckbriefe von See- und Wasservögeln der deutschen Nord- und Ostsee: Verbreitung, Ökologie und Empfindlichkeiten gegenüber Eingriffen in ihrem marinen Lebensraum. Naturschutz und Biologische Vielfalt Nr. 59, Bundesamt für Naturschutz, Bonn-Bad Godesberg (DEU)
Merkel B, Descamps S, Yoccoz NG, Grémillet D, Daunt F, Erikstad KE, Ezhov AV, Harris MP, Gavrilo M, Lorentsen SH, Reiertsen TK, Steen H, Systad GH, Þórarinsson PL, Wanless S, Strøm H (2021) Individual migration strategy fidelity but no habitat specialization in two congeneric seabirds. J Biogeogr 48(2):263–275
Moore FR, Woodrey MS, Buler JJ, Woltmann S, Simons TR (2005) Understanding the stopover of migratory birds: a scale dependent approach. In: Ralph C, John R, Terrell D (Eds) Bird Conservation implementation and integration in the Americas: Proceedings of the third international partners in flight conference. Pacific Southwest Research Station, USDA Forest Service General Technical Report PSW-191, Albany, Asilomar, California, USA, pp 684–689
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85(4):935–956
Newton I (2008) The migration ecology of birds. Academic Press, Oxford, UK
Newton I (2009) Moult and plumage. Ringing Migr 24(3):220–226
Newton I (2011) Migration within the annual cycle: species, sex and age differences. J Ornithol 152(1):169–185
Okill JD (1992) Natal dispersal and breeding site fidelity of red-throated divers Gavia stellata in Shetland. Ringing Migr 13(1):57–58
Okill JD (1994) Ringing recoveries of red-throated divers Gavia stellata in Britain and Ireland. Ringing Migr 15(2):107–118
Oppel S, Powell AN (2008) Assigning king eiders to wintering regions in the Bering sea using stable isotopes of feathers and claws. Mar Ecol Prog Ser 373:149–156
Paruk JD, Chickering MD, Long D, Uher-Koch H, East A, Poleschook D, Gumm V, Hanson W, Adams EA, Kovach KA, Evers DC (2015) Winter site fidelity and winter movements in common loons (Gavia immer) across North America. Condor: Ornithol Appl 117(4):485–493
Pennycuick CJ (1989) Bird flight performance: a practical calculation manual. Oxford Univ. Press, Oxford U.K
Peterson BJ, Frey B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Poessel SA, Uher-Koch BD, Pearce JM, Schmutz JA, Harrison AL, Douglas DC, Biela VR, Katzner TE (2020) Movements and habitat use of loons for assessment of conservation buffer zones in the Arctic coastal plain of northern Alaska. Glob Ecol Conserv. https://doi.org/10.1016/j.gecco.2020.e00980
QGIS Development Team (2018) QGIS geographic information system. Open Source Geospatial Foundation Project. http://qgis.osgeo.org
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Rappole JH (2013) The avian migrant: the biology of bird migration. Columbia University Press
Reed TE, Warzybok P, Wilson AJ, Bradley RW, Wanless S, Sydeman WJ (2009) Timing is everything: flexible phenology and shifting selection in a colonial seabird. J Anim Ecol 78:376–387
Ronconi RA, Swaim ZT, Lane HA, Hunnewell RW, Westgate AJ, Koopman HN (2010) Modified hoop-net techniques for capturing birds at sea and comparison with other capture methods. Mar Ornithol 38(1):23–29
Rubarth J, Dreyer A, Guse N, Einax JW, Ebinghaus R (2011) Perfluorinated compounds in red-throated divers from the German Baltic Sea: new findings from their distribution in 10 different tissues. Environ Chem 8(4):419–428
Runge CA, Martin TG, Possingham HP, Willis SG, Fuller RA (2014) Conserving mobile species. Front Ecol Environ 12(7):395–402
Rushing CS, Marra PP, Dudash MR (2016) Winter habitat quality but not long-distance dispersal influences apparent reproductive success in a migratory bird. Ecology 97(5):1218–1227
Ruthrauff DR, Tibbitts TL, Gill RE Jr (2019) Flexible timing of annual movements across consistently used sites by Marbled Godwits breeding in Alaska. Auk: Ornithol Adv. https://doi.org/10.1093/auk/uky007
Sæther BE, Bakke Ø (2000) Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81(3):642–653
Schmutz JA (2014) Survival of adult red-throated loons (Gavia stellata) may be linked to marine conditions. Waterbirds 37:118–124
Schreiber EA, Burger J (2001) Breeding biology, life histories, and life history–environment interactions in seabirds. Biology of marine birds. CRC Press, pp 235–280
Schwartz MD (1998) Green-wave phenology. Nature 394(6696):839–840
Shariatinajafabadi M, Wang T, Skidmore AK, Toxopeus AG, Kölzsch A, Nolet BA, Exo KM, Griffin L, Stahl J, Cabot D (2014) Migratory herbivorous waterfowl track satellite-derived green wave index. PLoS ONE. https://doi.org/10.1371/journal.pone.0108331
Skov H, Prins E (2001) Impact of estuarine fronts on the dispersal of piscivorous birds in the German bight. Mar Ecol Prog Ser 214:279–287
Smith JA, Regan K, Cooper NW, Johnson L, Olson E, Green A, Tash J, Evers DE, Marra PP (2020) A green wave of saltmarsh productivity predicts the timing of the annual cycle in a long-distance migratory shorebird. Sci Rep 10(1):1–13
Spiegel CS, Berlin AM, Gilbert AT, Gray CO, Montevecchi WA, Stenhouse IJ, Ford SL, Olsen GH, Fiely JL, Savoy L, Goodale MW, Burke CM (2017) Determining fine- scale use and movement patterns of diving bird species in federal waters of the mid- Atlantic United States using satellite telemetry. OCS Study BOEM, 69
St John Glew K, Wanless S, Harris MP, Daunt F, Erikstad KE, Strøm H, Speakman JP, Kürten B, Trueman CN (2019) Sympatric Atlantic puffins and razorbills show contrasting responses to adverse marine conditions during winter foraging within the North Sea. Mov Ecol 7(1):1–14
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8(11):1639–1644
Storer RW (1958) Loons and their wings. Evolution 12(2):262–263
Stresemann E, Stresemann V (1966) Die Mauser der Vögel. J Ornithol 107:3–448
Studds CE, Kyser TK, Marra PP (2008) Natal dispersal driven by environmental conditions interacting across the annual cycle of a migratory songbird. Proc Natl Acad Sci 105(8):2929–2933
Taylor CM, Norris DR (2010) Population dynamics in migratory networks. Thyroid Res 3:65–73
Thompson CW, Kitaysky AS (2004) Polymorphic flightfeather molt sequence in tufted puffins (Fratercula cirrhata): a rare phenomenon in birds. Auk 121:35–45
Todorov V, Filzmoser P (2009) An object-oriented framework for robust multivariate analysis. J Stat Softw 32(3):1–47
Trierweiler C, Klassen RHG, Drent RH, Exo KM, Komdeur J, Bairlein F, Koks BJ (2014) Migratory connectivity and population-specific migration routes in a long-distance migratory bird. Proc R Soc b: Biol Sci. https://doi.org/10.1098/rspb.2013.2897
Vardanis Y, Klaassen RH, Strandberg R, Alerstam T (2011) Individuality in bird migration: routes and timing. Biol Let 7(4):502–505
Vilela R, Burger C, Diederichs A, Nehls G, Bachl FE, Szostek L, Freund A, Braasch A, Bellebaum J, Beckers B, Piper W (2021) Use of an INLA latent Gaussian modelling approach to assess bird population changes due to the development of offshore wind farms. Front Mar Sci 8(882):1–11
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416(6879):389–395
Warnock N (2010) Stopping vs. staging: the difference between a hop and a jump. J Avian Biol 41(6):621-626.57
Wauchope HS, Shaw JD, Varpe Ø, Lappo EG, Boertmann D, Lanctot RB, Fuller RA (2017) Rapid climate-driven loss of breeding habitat for Arctic migratory birds. Glob Change Biol 23(3):1085–1094
Weber TP, Houston AI (1997) Flight costs, flight range and the stopover ecology of migrating birds. J Anim Ecol 66(3):297–306
Webster MS, Marra PP, Haig SM, Bensch S, Holmes RT (2002) Links between worlds: unraveling migratory connectivity. Trends Ecol Evol 17(2):76–83
Wetlands International (2019) Waterbird population estimates. http//wpe.wetlands.org. Accessed 23 Apr 2019
Wetlands International (2022) Waterbird populations portal. http//wpp.wetlands.org. Accessed 09 May 2022
Whitworth DL, Takekawa JY, Carter HR, Mciver WR (1997) A night-lighting technique for at-sea capture of Xantus’ Murrelets. Colon Waterbirds 20:525–531
Wilcove DS, Wikelski M (2008) Going, going, gone: is animal migration disappearing. PLoS Biol. https://doi.org/10.1371/journal.pbio.0060188
Winkler DW, Jørgensen C, Both C, Houston AI, McNamara JM, Levey DJ, Partecke J, Fudickar A, Kacelnik A, Roshier D, Piersma T (2014) Cues, strategies, and outcomes: how migrating vertebrates track environmental change. Mov Ecol 2(2):10
Žydelis R, Small C, French G (2013) The incidental catch of seabirds in gillnet fisheries: a global review. Biol Cons 162:76–88
Acknowledgements
We thank G. Guillou from the Plateforme Analyses Isotopiques of LIENSs laboratory for running the stable isotope analyses. Thanks are due to the CPER (Contrat de Projet Etat-Région) and the FEDER (Fonds Européen de Développement Régional) for funding the IRMS of LIENSs laboratory. The Institut Universitaire de France (IUF) is acknowledged for its support to PB as a Senior Member. Many thanks to Nadja Küpper and Yvonne Schumm for helpful discussion and support with the statistics. We greatly thank everybody involved in the capture of divers for their support. Thomas Grünkorn and Jorg Welcker completed our field team and supplied valuable support. Thomas Grünkorn also contributed by ringing of the captured red-throated divers. Sören Zenner (OS-Energy) allowed flexible organisation and provision of ships during the field seasons and we thank the ship captains and crews of the MS Madog and MS Arctic Hunter for their reliability and support during fieldwork. Finally, we thank the reviewers for their valuable recommendations.
Funding
Open Access funding enabled and organized by Projekt DEAL. The DIVER project is supported by the Federal Ministry for Economic Affairs and Energy on the basis of a decision by the German Bundestag (funding ID 0325747 A/B).
Author information
Authors and Affiliations
Contributions
All authors were involved either field work, lab work or in the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors explicitly declare that they have no competing interests.
Ethical approval
The ethical rules as well as the legal requirements for the fieldwork have been met. All fieldwork (animal capture, sampling and tagging) was approved by BfN (Federal Agency for Nature Conservation, Germany, 05.08.2014; and Ministry of Environment and Food Denmark, Danish Veterinary and Food Administration, permit no. 2014-15-0201-00239, issued 18.12.2014).
Consent for publication
All co-authors have seen and agreed with the contents of the manuscript for publication.
Additional information
Responsible Editor: T.A. Clay.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kleinschmidt, B., Burger, C., Bustamante, P. et al. Annual movements of a migratory seabird—the NW European red-throated diver (Gavia stellata)—reveals high individual repeatability but low migratory connectivity. Mar Biol 169, 114 (2022). https://doi.org/10.1007/s00227-022-04096-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04096-x