Abstract
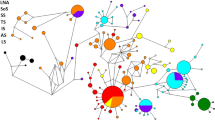
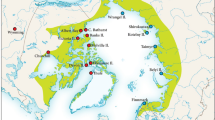
The purpose of this study was to determine the phylogeographic structure of the brackish-hypersaline cyprinodont fish Aphanius fasciatus (Valenciennes, 1821), using sequencing and RFLP analysis of a 1,330 bp mitochondrial DNA segment containing part of the 16S rRNA gene as well as the genes for tRNA-Leu, NADH subunit 1 and tRNA-Ile. Individuals were collected from 13 different sites in Greece and Turkey, while seven published A. fasciatus sequences were also included to cover the area of distribution of the species. Pairwise sequence divergence values ranged from 0 to 4.51%. Congruent phylogenies were recovered with maximum likelihood, maximum parsimony and neighbour-joining methods. All analyses revealed two main groups. The first group consists of populations from almost all localities that drain into the Aegean Sea. The second group comprises the remaining population samples, which in some cases seem to consist of population-specific subgroups. Our results show that vicariant events have predominantly affected the evolution of A. fasciatus, with the Messinian salinity crisis having shaped the present genetic structure of its populations. Additionally, the life-history traits of the species, which determine a low potential for dispersal, coupled with the typical fragmentation of brackish-hypersaline water habitats have led to a high degree of isolation of A. fasciatus populations, even at restricted spatial scales. Analysis of the partitioning of the total amount of polymorphism with analyses of molecular variance (AMOVA) gave a value of F ST = 84.6%. Potential conservation policies concerning A. fasciatus should also consider the low-genetic variability in the majority of its populations and the presence of fixed haplotypes in some of them.


Similar content being viewed by others
References
Abbiati M, Maltagliati F (1996) Allozyme evidence of genetic differentiation between populations of Hediste diversicolor (Polychaeta: Nereididae) from the Western Mediterranean. J Mar Biol Assoc UK 76:637–647
Ashbaugh NA, Echelle AA, Echelle AF (1994) Genetic diversity in Red River pupfish Cyprinodon rubrofluviatilis (Atheriniformes, Cyprinodontidae) and its implications for the conservation genetics of the species. J Fish Biol 45:291–302
Battaglia B, Bisol PM, Fava G (1978) Genetic variability in relation to the environment in some marine invertebrates. In: Battaglia B, Beardmore J (eds) Marine organisms: genetics, ecology and evolution. Plenum Press, New York, pp 53–70
Baxevanis AD, Kappas I, Abatzopoulos TJ (2006) Molecular phylogenetics and asexuality in the brine shrimp Artemia. Mol Phylogenet Evol 40:724–738
Bianco PG (1990) Potential role of the paleohistory of the Mediterranean and Paratethys basins on the early dispersal of Euro-Mediterranean freshwater fishes. Ichthyol Explor Freshw 1:167–184
Birky CW, Maruyama T, Fuerst P (1983) An approach to population and evolutionary genetic theory for genes in mitochondria and chloroplasts and some results. Genetics 103:513–527
Boudouresque CF (1996) Impact de l’homme et conservation du milieu marin en Méditerranée. GIS Posidonie publications, Marseille
Carvalho GR (1993) Evolutionary aspects of fish distribution: genetic variability and adaptation. J Fish Biol 43(Suppl A):53–73
Cimmaruta R, Scialanca F, Luccioli F, Nascetti G (2003) Genetic diversity and environmental stress in Italian populations of the cyprinodont fish Aphanius fasciatus. Oceanol Acta 26:101–110
Cognetti G (1994) Colonization of brackish waters. Mar Pollut Bull 28:583–586
Cognetti G, Maltagliati F (2000) Biodiversity and adaptive mechanisms in brackish water fauna. Mar Pollut Bull 40:7–14
Crivelli AJ (2005) Aphanius fasciatus. In: IUCN 2006. 2006 IUCN Red List of Threatened Species, www.iucnredlist.org
Doadrio I, Perdices A, Machordom A (1996) Allozymic variation of the endangered killifish Aphanius iberus and its application to conservation. Environ Biol Fish 45:259–271
Dunham JB, Minckley WL (1998) Allozymic variation in desert pupfish from natural and artificial habitats: genetic conservation in fluctuating populations. Biol Conserv 84:7–15
Excoffier L, Smouse P, Quattro J (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 131:479–491
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evol Bioinformatics Online 1:47–50
Felsenstein J (1985) Confidence limits on phylogenies. An approach using the bootstrap. Evolution 39:783–791
Ferrito V, Maltagliati F, Mauceri A, Adorno A, Tigano C (2003) Morphological and genetic variation in four Italian populations of Lebias fasciata (Teleostei, Cyprinodontidae). Ital J Zool 70:115–121
Ferrito V, Mannino MC, Pappalardo AM, Tigano C (2007) Morphological variation among populations of Aphanius fasciatus Nardo, 1827 (Teleostei, Cyprinodontidae) from the Mediterranean. J Fish Biol 70:1–20
Fischer W, Schneider M, Bauchot ML (1987) Fiches FAO d’identification des espèces pour les besoins de la pêche. Méditerranée et Mer Noire, Zone de pêche 37, Rev. I, vol II Vertèbres. FAO CEE, Rome, pp 761–1529
Garcia-Marin JL, Pla-Zanuy C (1999) Conservacion de la diversidad genetica en el fartet, L. ibera. In: Planelles-Gomis M (ed) Peces Ciprinodontidos Ibericos Fartet Y Samaruc Generalitat Valenciana. Conselleria de Medio Ambiente, Valencia, pp 169–187
Garcia-Marin JL, Vila A, Pla C (1990) Genetic variation in the Iberian toothcarp Aphanius iberus (Cuvier and Valenciennes). J Fish Biol 37(Suppl A):233–234
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Gyllensten U (1985) The genetic structure of fish: differences in the intraspecific distribution of biochemical genetic variation between marine, anadromous, and freshwater species. J Fish Biol 26:691–699
Hartl DL, Clark AG (eds) (1997) Principles of population genetics. Palgrave Macmillan Publishers Houndmills, Basingstoke, England
Hillis DM, Moritz C, Mable BK (eds) (1996) Molecular systematics, 2nd edn. Sinauer Associates, Sunderland MA
Hrbek T, Meyer A (2003) Closing of the Tethys Sea and the phylogeny of Eurasian killifishes (Cyprinodontiformes: Cyprinodontidae). J Evol Biol 16:17–36
Hsü KJ, Montadert L, Bernoulli D, Cita MB, Erickson A, Garrison RE, Kidd RB, Mèlierés F, Müller C, Wright R (1977) History of the Mediterranean salinity crisis. Nature 267:399–403
Kiener A, Schachter D (1974) Polymorphisme d’ Aphanius fasciatus Nardo, 1827 (Poisson Cyprinodontidae) des eaux saumâtres (Populations de Corse et de la lagune italienne de Comma Chio). B Mus Natl Hist Nat 142:317–339
Kirchhoff S, Sévigny JM, Couillard CM (1999) Genetic and meristic variation in the mummichog, living in polluted and reference estuaries. Mar Environ Res 47:261–283
Kraiem MM (1983) Les poissons d’eau douce de Tunisie: inventaire comment et répartition géographique. Bull Inst Natl Sci Tech Océanogr Pêche Salammbô 10:107–124
Krieg F, Triantafyllidis A, Guyomard R (2000) Mitochondrial DNA variation in European populations of Silurus glanis. J Fish Biol 56:713–724
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Leberg PL (1992) Effect of population bottlenecks on genetic diversity as measured by allozyme electrophoresis. Evolution 46:477–494
Leonardos ID (1996) Population dynamics of toothcarp (Aphanius fasciatus Nardo, 1827) in the Mesolongi and Etolikon lagoons. University of Thessaloniki: Dissertation thesis (in Greek)
Leonardos I, Sinis A (1998) Reproductive strategy of Aphanius fasciatus Nardo, 1827 (Pisces: Cyprinodontidae) in the Mesolongi and Etolikon lagoons (W. Greece). Fish Res 35:171–181
Leonardos I, Sinis A (1999) Population age and sex structure of Aphanius fasciatus Nardo, 1827 (Pisces: Cyprinodontidae) in the Mesolongi and Etolikon lagoons (W. Greece). Fish Res 40:227–235
Leonardos I, Sinis A, Petridis D (1996) Influence of environmental factors on the population dynamics of Aphanius fasciatus (Nardo, 1827) (Pisces: Cyprinodontidae) in the lagoons Messolongi and Etolikon (W. Greece). Isr J Zool 42:231–249
Maltagliati F (1998a) A preliminary investigation of allozyme genetic variation and population geographical structure in Aphanius fasciatus from Italian brackish-water habitats. J Fish Biol 52:1130–1140
Maltagliati F (1998b) Does the Mediterranean killifish Aphanius fasciatus (Teleostei: Cyprinodontidae) fit the one-dimensional stepping-stone model of gene flow? Environ Biol Fish 53:385–392
Maltagliati F (1999) Genetic divergence in natural populations of the Mediterranean brackish-water killifish Aphanius fasciatus. Mar Ecol Prog Ser 179:155–162
Maltagliati F (2002) Genetic monitoring of brackish-water populations: the Mediterranean toothcarp Aphanius fasciatus (Cyprinodontidae) as a model. Mar Ecol Prog Ser 235:257–262
Muus BJ (1967) Some problems facing the ecologist concerning races and subspecies of brackish-water animals. In: Lauff GH (ed) Estuaries. American Association for the Advancement of Science, Washington DC, pp 558–563
Naihong X, Audenaert E, Van Overbeke J, Brendonck L, Sorgeloos P, De Meester L (2000) Low among-population genetic differentiation in Chinese bisexual Artemia populations. Heredity 84:238–243
Parenti LR, Tigano C (1993) Polymorphic skeletal characters in Aphanius fasciatus Teleostei: Cyprinodontiformes. Copeia 4:1132–1137
Perdices A, Carmona JA, Fernandez-Delgado C, Doadrio I (2001) Nuclear and mitochondrial data reveal high genetic divergence among Atlantic and Mediterranean populations of the Iberian killifish Aphanius iberus (Teleostei: Cyprinodontidae). Heredity 87:314–324
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818
Rice WR (1989) Analysing tables of statistical tests. Evolution 43:223–225
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tigano C, Canapa A, Ferrito V, Barucca M, Arcidiacono I, Olmo E (2004) Osteological and molecular analysis of three Sicilian populations of Aphanius fasciatus (Teleostei, Cyprinodontidae). Ital J Zool 71:107–113
Tigano C, Canapa A, Ferrito V, Barucca M, Arcidiacono I, Deidum A, Schembri PJ, Olmo E (2006) A study of osteological and molecular differences in populations of Aphanius fasciatus Nardo 1827 from the central Mediterranean (Teleostei, Cyprinodontidae). Mar Biol 149:1539–1550
Torchio M (1967) Osservazioni e considerazioni sulla presenza in acque mediterranee costiere di ciprinidi, ciprinodontidi e gasterosteidi. Natura: Rivista di Scienze Naturali 58:235–243
Triantafyllidis A, Abatzopoulos TJ, Economidis PS (1999) Genetic differentiation and phylogenetic relationships among Greek Silurus glanis and Silurus aristotelis (Pisces, Siluridae) populations, assessed by PCR-RFLP analysis of mitochondrial DNA segments. Heredity 82:503–509
Triantaphyllidis GV, Criel GRJ, Abatzopoulos TJ, Thomas KM, Peleman J, Beardmore JA, Sorgeloos P (1997) International study on Artemia. LVII. Morphological and molecular characters suggest conspecificity of all bisexual European and North African Artemia populations. Mar Biol 129:477–487
Tsigenopoulos CS, Durand JD, Ünlü E, Berrebi P (2003) Rapid radiation of the Mediterranean Luciobarbus species (Cyprinidae) after the Messinian salinity crisis of the Mediterranean Sea, inferred from mitochondrial phylogenetic analysis. Biol J Linn Soc 80:207–222
Vrijenhoek RC, Douglas ME, Meffe GK (1985) Conservation genetics of endangered fish populations in Arizona. Science 229:400–402
Ward RD, Woodwark M, Skibinski DOF (1994) A comparison of genetic diversity levels in marine, freshwater, and anadromous fishes. J Fish Biol 44:213–232
Whitehead P, Bauchot JP, Hureau ML, Nielsen JC, Tortonese EJ (eds) (1986) Fishes of the North-Eastern Atlantic and the Mediterranean, vol II. Unesco, Paris
Acknowledgements
We thank Dr. A. D. Baxevanis, Dr. H. Sari, Mrs. R. Barbieri, Dr. E. Kalogianni and Mrs. E. Dimitriou for their assistance in collecting the fish samples used in the present study. We also thank S. Papakostas for his help in statistical analyses as well as two anonymous reviewers for their valuable comments. We declare that all experiments comply with the current European Union laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne.
Rights and permissions
About this article
Cite this article
Triantafyllidis, A., Leonardos, I., Bista, I. et al. Phylogeography and genetic structure of the Mediterranean killifish Aphanius fasciatus (Cyprinodontidae). Mar Biol 152, 1159–1167 (2007). https://doi.org/10.1007/s00227-007-0760-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0760-7