Abstract
Disaggregating seagrass meadows and studying its components separately (clones, ramets, shoots) can provide us insights on meadow dynamics and growth patterns. The clonal growth, dependent upon clonal rules may regulate and impose constraints to plant architecture and, therefore, determine how individual clones evolve into the environment. In order to investigate the relationship between clonal growth rules and clone architecture, the belowground network architecture of single-clones of the seagrass Zostera noltii was studied. Networks were traced in situ after washing out the overlying sediment, and network characteristics were measured using digital analysis: area covered by clone, total rhizome length, type of rhizomatic axes (main, secondary, tertiary, quaternary), number and length of the internodes, branching angles and branching frequencies. This approach revealed that Z. noltii is able to develop into large clones integrating up to 300 internodes, 676 cm of rhizome, 208 shoots and 4,300 cm2 of plant area. Internodal length depended on both, the distance to the apical shoot (time effect) and the axes type (apical dominance effect). However, average branching angle was independent of axis type (average 58.3 ± 0.75), but varied significantly depending on the distance from the apical shoot. This average branching angle allows Z. noltii maximize the rate of centrifugal expansion, maintaining a high density in colonized areas to produce close stands but also minimizing the investment in belowground biomass and ramets overlapping. The clonal architecture of Z. noltii seems to be regulated by the interaction of both, apical dominance strength and clonal integration distance. Moreover, clonal growth rules and growth pattern seem to constrain clonality through (clonal) plant architecture regulations (i.e. branching is restricted in secondary axes, similar average branching angles regardless the axes, the higher the distance to the apex the higher the number of internodes in secondary axes, shorter internodes in secondary and tertiary axes). Future research efforts should focus on how these complex relationships between apical dominance and clonal integration interact to elucidate the temporal (seasonal) and spatial scales of both processes and the outcome at the plant architectural level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last years, there is a renewed interest in the ecology and evolution of plastic variability in highly modular organisms like seagrasses and other clonal plants (Kendrick et al. 2005; de Kroon et al. 2005). There are two main reasons for such renovated attention: (a) the availability of new genetic tools in ecology allow to retake questions regarding clonal and genetic variability in plants (Coyer et al. 2004a, b; Ruggiero et al. 2005a; Reusch 2006); and (b) the accessibility to powerful computers for a more realistic and complex simulations of clonal growth (Marbá and Duarte 1998; Prusinkiewicz 2004; Magyar et al. 2004; Sintes et al. 2005; Brun et al. 2006b). Clonality may be viewed as a simple way of growth, where plants are arranged by repetition of a fundamental unit, the phytomer (sensu Briske 1991 and Brun et al. 2006a). Moreover, environmental variability can be somewhat managed throughout the large architectural and functional variability driven by clonal growth (Short 1983; Peralta et al. 2005; Brun et al. 2006b) because the plant architecture is relatively plastic and the interaction with the environment adjusts the actual plant form (Oborny 2004). In addition, there are two major processes that modulate the final clone architecture: (1) apical dominance and, (2) clonal integration. Apical dominance is used to denote the correlative influence of the apex, or dominant shoot, on the growth and orientation of lateral organs (Woolley and Wareing 1972; reviewed in Phillips 1969). Apical dominance seems to be mediated by the release of hormones at the apex level (Harrison and Kaufman 1980; Cline 1996), although other studies also reported the involvement of the roots in addition to the apical shoot (Harrison and Kaufman 1984; Cline 1997). On the other hand, clonal integration allows connected units (shoots, rhizomes and roots) to share resources, and individual clones to feed from different microenvironments simultaneously (Groenendael and de Kroons 1990; Cain 1994).
A large seagrass meadow can be viewed as a static photograph of a longer movie that started long time ago. The settlement of a seedling or propagule that begins to spread is the initial starting point of this history. Beyond, seagrass meadows are highly dynamic ecosystems with several processes interacting at different spatio-temporal scales (Duarte et al. 2006). Dynamic processes and factors affecting the largest scale (i.e. seagrass meadows) have been thoroughly studied in seagrass literature (see references in Duarte et al. 2006 and Bell et al. 2006). However, seagrass meadows are formed by the reiteration of small units, the clone, which is built by ramets and thus by the repetition of the simplest unit, the “phytomer” (Briske 1991; Brun et al. 2006a). As it was recently expressed by Duarte et al. (2006), the proper understanding of the different processes affecting the dynamic of seagrass ecosystems needed a basic understanding on the contribution of the different relevant processes involved in clonal growth. That is, from the dynamic of apical meristems and the resultant shoots, to that of the patches. However, studies on factors affecting to this level of organization (individual clone), as for instance, apical dominance (Terrados et al. 1997; Marbá et al. 2002), clonal integration (Tomasko and Dawes 1989; Marbá et al. 2002) or the growth of individual clones (Brouns 1987; Brun et al. 2006a) are rare on seagrass literature. Recently, it has been demonstrated that plant morphology arises as an emergent property of a simple set of growth rules acting at the module level, which depicts differences among species in plant morphology and meadow properties (De Kroon et al. 2005; Brun et al. 2006b). Furthermore, other modelling approaches (Marbá and Duarte 1998; Sintes et al. 2005, 2006) have stated the critical role of the rhizome branching angle and frequency in determining the growth of the clonal network, and the emergence of non-linear behaviour in patch growth dynamics. Main differences between both modelling approaches are that the former uses the “phytomer”-environment interactions in a continuous feedback to build the clone morphology and the resulting meadow. It therefore, accounts for the biotic and abiotic heterogeneities generated throughout the clone evolvement. Contrastingly, the latter approach uses a combination of architectural (measured clonal growth rules) and dynamic data (shoot mortality) to simulate meadow growth. Both approaches have been successful in explaining different ecological and dynamic traits of seagrasses. However, in order to develop complex functional-structural seagrass models (such as that proposed in Brun et al. 2006b) a detailed description of the development of individual clones is necessary to (1) improve our understanding on the interplay between clonal rules and the resulting plant dynamic and architectural features, (2) acquire input data for the models, and (3) obtain a large pool of data to check the output from models.
As it was stated above, branching frequency and angle of the rhizome network seem to be the key factors switching the colonizing strategy from phalanx (i.e clumped growth) to guerrilla (i.e. runner growth) type of growth (Lovett-Doust 1981; Callaghan et al. 1990; Sintes et al. 2005). Branching angles and internodal lengths are somewhat variables (Duarte 1991; Marbá and Duarte 1998) and probably tuned by individuals in response to spatial and temporal heterogeneity in resources (see review table in Brun et al. 2006b). Therefore, several important ecological traits related with the colonizing strategy (de Kroons and Knops 1990; Sintes et al. 2005), mating development (Charpertier 2002; Ruggiero et al. 2005a, b), competition capacity (Stoll et al. 1998; Oborny et al. 2000) and foraging behaviour (de Kroons and Schieving 1991; Oborny et al. 2001), depend largely on how species allocate biomass into belowground networking. However, there is no data available on within-clone variability and/or the dependence of branching angle and internodal length with clonal rules.
Thus, the main goal of this study was to describe the variability of the clonal network characteristics (branching angle and frequency, internodal number and length, and clone size) in individual clones of the “phalanx” seagrass Zostera noltii, and to relate this variability with clonal growth rules (apical dominance and clonal integration). The seagrass Z. noltii was chosen because it is a fast growing species that develops branching at every node and, therefore, it may reflect, at short temporal and spatial scales, a high variability in the studied features.
Materials and methods
Sampling site
This study was conducted in an intertidal sandflat at Los Toruños (el Bajo de la Cabezuela), a salt marsh ecosystem of 773 Ha situated in the Cadiz Bay Natural Park (Southern Spain, 36°30′N, 6°10′W). This sandflat, located at the mouth of a tidal channel, undergoes strong tidal currents, waves and winds with recurrent episodes of sediment erosion and accretion (see Brun et al. 2005 for further information). Patches of the seagrass Zostera noltii Hornem. occur in the intertidal areas, whereas beds of Cymodocea nodosa (Ucria) Ascherson and the macroalgae Caulerpa prolifera (Forsskäl) Lamouroux thrive in the lower intertidal and shallow subtidal zones.
Plant sampling
The study was performed in spring (April–May 2005) when small patches of the seagrass Zostera noltii started to colonize this area. During summer the coalescence of the neighbouring patches often results into widespread meadows. In autumn–winter, the extensive meadows underwent fragmentation as a consequence of the increment of storm-driven currents (Brun et al. 2005). To investigate the physical clonal extent of the Z. noltii plants (i.e. plant size) and the within-plant network characteristics (i.e. internodal length, rhizome branching angle and frequency), up to 50 isolated clones were selected randomly along the intertidal sandflat. However, only those single clone-patches (i.e. those derived from one single plant) were selected after a careful sediment removal (see below). Plants occurring at larger patches were ignored in a try to reduce density-dependence phenomenon. Furthermore, those clones where the belowground network was not intact were also ignored, because we cannot guarantee that they bore from one single clone. Overlying sediment was washed out carefully with a water pump to show the rhizome network up, while keeping it unaltered in its natural position (rhizomatic structures sometimes were buried up to 5 cm depth). Once the whole (intact) belowground matrix was patent, it was traced with a waterproof pen in a transparent film placed on it (nodes, internodes, nodes bearing shoots and apical shoots were drawn).
Plant analysis
Once individual clones were depicted in the field, the overheads were scanned and digitally analyzed in the laboratory as follows. First, all the internodes within each rhizome axis (or ramet) were numbered in ascendant from the apical shoot located in the apex of the rhizome axis (node zero). Furthermore, all ramets were sorted out hierarchically: 1 (main ramet), 1.1 (secondary ramets), 1.1.1 (tertiary ramets), 1.1.1.1 (quaternary ramets; Fig. 1). Internodal lengths and branching angles were determined by “ImageJ v.10.2” software. Total ramet length (depending on its degree; primary, secondary, tertiary and quaternary) was calculated by summing all the within-ramet internodal lengths. The branching angle was determined between intersecting axes (i.e. main–secondary, secondary–tertiary, tertiary–quaternary) close to the point where they met (Fig. 1). Total plant area was calculated from the digitalized images assuming an ellipsoidal shape and using the largest main and secondary axes as the longest and the shortest axes of the ellipse.
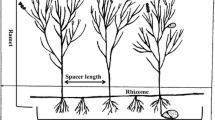
Zostera noltii. Schematic illustration of an individual clone with the main architectural features of the clone. Lines represent the internode, while arrows indicate apical shoots. P primary ramet, S secondary ramet, T tertiary ramet, Q quaternary ramet. Numbers indicate how nodes were numbered in an ascendant and hierarchical mode from the apical shoot (node zero).œ denotes the branching angle between P–S ramets, ß denotes the branching angle between S–T ramets, π denotes the branching angle between T–Q ramets
Considering that the clonal growth of Zostera noltii proceeds by the regular addition of new modules at the apex of the main and secondary rhizomes (or tertiary; Brun et al. 2006a), the position of every node in relation to the apical shoot sets a time scale from the present (node zero—apical shoot—) back to the past (node n + i). It allows a comparison of the internodal length and branching angle at different node positions among different axes. Indeed, as internode growth progresses during one plastochrone interval in the apical shoots (Brun et al. 2006a), the internode position was multiplied by an annual averaged leaf plastochrone interval (8.3 ± 0.67 days, n = 959; Brun et al. 2003, 2006b) to convert node position into age.
Statistics
Belowground morphometric differences were tested by one-way ANOVA, while a two-way ANOVA test was used to check differences between main and secondary axes in the number of total internodes as a function of the number of internodes in the axis (axis and number of internodes in the axis as independent factors). Homogeneity of variances and normality of data were previously tested and data were log-transformed when it was necessary to comply with homocedasticity and normality. Multiple post hoc comparisons between means were assessed by the Tukey procedure (Zar 1984). Sequential Bonferroni correction (Holm 1979) and the truncated product method (Zaikin et al. 2002) were applied in a try to reduce the type I error, but also to avoid the increment in type II error (Moran 2003; Neuhäuser 2004). Allometric scaling, i.e., the dependence of architectural and dynamic properties on plant size was studied by fitting the dataset to the equation Y = a·Xb . This procedure has been extensively used in several disciplines such as biology, plant science and seagrass ecology (Duarte 1991; Niklas 1994; West et al. 1999). The coefficient of determination (R2) was used to describe the percent of the variation that is explained by the regression equation, while their precision is estimated by the standard error of the regression. In all cases, the significance level was set at 5% probability. Data are presented as mean ± 1 standard error.
Results
Belowground morphometric features
The main morphometric features of the rhizome network are shown in Table 1. A descriptive example of both extremes in clonal topologies of Zostera noltii plants found at the sampling zone is shown in Fig. 2. The clone size was highly variable: from small plants (24 cm long, 42 cm2 of plant area) formed by 15 internodes and simply secondary ramets (Table 1; Fig. 2a) up to larger plants (675 cm long, 4,300 cm2 of plant area) having up to 300 internodes and quaternary ramets (Table 1; Fig. 2b, c). The number of internodes in the main axis ranged between 7–29 internodes plant−1, equivalent to an average and maximum plant age of 129 ± 9 and 241 days, respectively. A large inter- and intra-plant variability was recorded for internodal length and branching angle: from short internodes and acute branching angles (minimum 0.28 cm and 11.8°) to larger internodes and obtuse branching angles (maximum 6.39 cm and 153°). In addition, significant differences were found in the internodal length when the axis type (primary, secondary, etc) was considered (Table 2; Fig. 3a), whereas no significant differences were recorded for the branching angle (Table 2). A similar average branching angle (average 58.3 ± 0.75, n = 738) was recorded independently of the axis type (Table 1). Additionally, significant differences in the internodal length and branching angle in the main rhizome axis were observed depending on the node position in relation to the apical shoot (i.e. the longer the distance between the apical shoot and a given node, the shorter the internodes and the wider the branching angles). Comparisons of the internodal length between main and secondary axes located at the same position (in relation to the apical shoot) did reveal significant differences up to node n° 5, while no significant differences were recorded beyond this node (with the exception of node 13; Fig. 3b). When plotted against clone area (m2) using the allometric equation, the slope was higher for total rhizome length (TRL; slope = 0.78 ± 0.04) than for the total number of shoots (TNS; slope = 0.68 ± 0.04). In contrast, shoot density decreased with increasing plant size (Fig. 4; Table 3).
Zostera noltii. Variability in network features when the factors “time” (position from the node 0) and “apical dominance” (different axes) are examined. a Internodal length in the different axes (primary, secondary, tertiary and quaternary). b Internodal length in primary, secondary and tertiary axes when the factor “time” is examined. c Branching angle in primary and secondary axes when the factor “time” is examined. Data are presented as mean ± 1 SE. Different letters indicate significant differences (P < 0.05)
Zostera noltii. Relationships between total rhizomatic length (TRL open circles), total number of shoots (TNS closed circles) and shoot density (cross symbols) with clone size. Lines denote the fitting to the allometric equation of the form Y = a·Xb. Regression parameters and statistical results are given in Table 3. See statistical section for further information
Clonal growth pattern
A detailed analysis of the belowground network revealed a highly organized pattern of branching and growth. First, all branches (or ramets) fulfil the branching pattern described previously for this species, that is, the first axillary shoot branches in the opposite direction than the previous branch (Brun et al. 2006a). Secondly, branching frequencies increase quickly from node 0 (branching probability is zero) to node 4 (branching probability is above 95%), while branching frequencies beyond the node 13 diminishes substantially (Fig. 5) As well, the number of internodes in secondary axes fitted to a bell-shape curve, with a gradual increase from node 2 up to node 15. Afterwards there was a reduction in the number of internodes in the secondary axis (Fig. 5). Moreover, there was a linear increase at a constant rate of 0.3 (R2 = 0.98, df = 27, F = 1,748, P < 0.0001) between the number of branches and the total number of internodes per plant, yielding a new branch approximately every 3 new internodes produced (data not shown). Compared to the primary axis, branching of secondary axes was constrained (two-way ANOVA, Table 2) when the total number of internodes (TNI; summing internodes placed at branches) were computed as a function of the number of internodes in the axis (NIA; either primary or secondary, Fig. 6). Furthermore, the slope of the allometric scaling regression for the total number of internodes in primary axes was higher than that recorded for secondary axes (1.72 ± 0.03 and 1.29 ± 0.02, respectively; Fig. 6; Table 3). Although up to 17 internodes were recorded in secondary axes (Table 1), values higher than 13 were disregarded because only one case was recorded.
Zostera noltii. Total number of internodes in the clone as function of the number of nodes in the main (closed circles) or secondary (open circles) axis. Data are represented as mean as ± 1 SE. Lines represent the fitting to the allometric equation of the form Y = a·Xb . Regression parameters and statistical results are given in Table 3. See statistical section for further information
Discussion
This study reveals that clonal architecture of the seagrass Zostera noltii is a tightly regulated process, but displaying an inherent variability (Table 1). Moreover, it is noticeable that clonal growth rules and patterns may constrain the clonal behaviour in this species by regulating the plant architectural features. That is, frequency of branching in secondary axes is restricted; thus, the longer the distance to the apex the higher the number of internodes. Moreover, the average branching angle is nearly identical regardless the axis type; thus, the higher the axis type (degree) the shorter the internodes. Although this is the first report on seagrass clonal constraints, it is acknowledged that clonality in land plants may impose limits on within-plant resource allocation patterns (Armstrong 1983; Schmid et al. 1988). Our results show both that apical dominance and clonal integration may play a key role in the clonal architecture of Z. noltii. Apical dominance occurs through the hormonal control on the bud primordium exerted by the apical shoot (Harrison and Kaufman 1984; Cline 1996). It has been demonstrated experimentally for a limited number of seagrass species. Terrados et al. (1997) reported that the experimental decapitation of the apex of a Cymodocea nodosa clone did increase the branching frequency up to 50 cm far away from the excised apex. Our observations suggest that apical dominance, besides controlling bud outgrowing (restricting branching in secondary axes or ramets; Fig. 6) may regulate the internodal length in daughter ramets (Fig. 3a).
It has been proposed that an additional mechanism of apical dominance may be the control of the resource partitioning between main axis and branches stemming from it (i.e. “nutrient diversion theory” sensu Phillips 1975; McIntyre 1977). This hypothesis predicts that apical shoots can act as strong metabolic sinks driven by its high growth rates and hormone levels that would deprive nutrients from branches reducing their growth. The use of carbon and/or nitrogen isotopes has revealed resource translocation within seagrass plants (Harrison 1977; Libes and Bodouresque 1987; Tomasko and Dawes 1989; Zimmerman et al. 1995; Marbá et al. 2002). However, only Marbá et al. (2002) included the apical dominance as a correlative process in the experiment, demonstrating that the translocation of new photosynthates proceeded mostly towards the apical shoot of the main axis (Fig. 5 in Marbá et al. 2002). Nevertheless, these authors did not discriminate between main and secondary axes. Overall, results recorded by Marbá et al. (2002) did indicate a preferential allocation of newly acquired resources towards apical parts, draining the resources in secondary and tertiary axes, which may entail a reduction in growth and internodal lengths (Wilson 2000).
Considering average internodal lengths for different axis types in Zostera noltii (Table 1) and the maximum transport distance recorded in this species (approximately 25 cm for 15N and 13C; Marbá et al. 2002), it yields that the primary axis is fully integrated up to internode number 8, whereas up to 12 internodes are integrated in secondary axes. Using mean internodal lengths (primary and secondary axes), branching angles (Table 1) and the number of shoots stemming from secondary axes (Fig. 5) it can be depict the theoretical portion of a Z. noltii plant that is fully integrated. This approach yields that up to 22 shoots (1 main apical, 7 secondary apical shoots and 14 lateral shoots), 28 internodes, 70 cm of rhizome and 460 cm−2 of plant area may be clonally integrated sharing resources. Moreover, some of the intermediate shoots in the main and secondary axes can be controlled by at least two different apical shoots and, even the apical shoot s of secondary axes, may be under the influence of the main apical and other secondary apical shoots. Furthermore, the results using this approach also match with those shown in Fig. 3b, where significant differences in internodal length between main and secondary axes were recorded up to node number 4 in secondary ones. It might indicate that new shoots and internodes growing beyond this node have “escaped” from the main apical dominance. If the balance between apical dominance strength and clonal integration accounts for clonal architecture in this species, the knowledge of the factors regulating both processes will be essential in understanding how plant evolves in the environment and consequently at the largest scale (seagrass meadow). Apical strength seems to be negatively correlated with light (Irwin and Aarssen 1996) and nutrient availability (McIntyre 1973), whereas resource translocation seems to depend also on light conditions (Zimmerman et al. 1995) and ambient nutrient availability (Li et al. 1992). However, the understanding of the interplay of these complex relationships (i.e. apical dominance, clonal integration and clone architecture) in Z. noltii needs further investigations, including temporal and spatial scales in both processes.
A foremost implication of clonal integration in plants with numerous shoots is that these plants feed from different (micro) environments and share acquired resources among shoots, favouring the apical translocation. Furthermore, the clonality may allow individual shoots to buffer local environmental conditions (low light or nutrient deficiency) as was demonstrated in the seagrass Thalassia testudinum (Tomasko and Dawes 1989). Another important implication is the parallel increase in shoot recruitment with clone size and, hence, in the number of “resource-supplying units” to the main apices. It may involve positive feedbacks in the main apical shoots, increasing the rhizome elongation and shoot recruitment rates, which may account for the non-linearity growth process observed in some seagrass species (Vidondo et al. 1997; Kendrick et al. 2000; Sintes et al. 2005). However, and according to our data (Fig. 4; Table 3), this advantage can turn into a drawback, since the number of shoots increases with plant size at a lower rate than rhizomes do. It would reduce the above-belowground biomass ratio to critical values when plant size increases greatly, reducing the carbon balance in non-photosynthetic tissues and increasing the risk of negative cascade effects if light conditions deteriorates (Hemminga 1998; Peralta et al. 2005, 2006). Density-dependent phenomena that may also account for this behaviour (reduced branching rate and rhizome length in secondary axes) must be discarded in this study, since clones were isolated and shoot density did not increase with clone size (even a slight reduction was recorded; Fig. 4).
Branching angle is considered a key factor in determining the rate of centrifugal spreading in clonal species (Smith and Palmer 1976; Callaghan et al. 1990; Sintes et al. 2005). For instance, Smith and Palmer (1976) recorded that plant development used to undertake a geometric model. Accordingly, they pointed out that an optimal pattern of stem production, i.e., that which maximizes the stem density in colonized areas to produce a closed stand (thereby avoiding intrusion of competitive clones) but with a minimum investment in belowground system, will be obtained if ramets branch at a 60° divergent angle from mother ramets (one ramet growing to the left and the contiguous one to the right side). Furthermore, branches branching at 60° divergent angle from mother ramets need of some variability in the branching angle, and that some ramets branch in the same direction to the mother branch, to fill the gaps produced by this structure (Smith and Palmer 1976; Callaghan et al. 1990; Meyer and Schmid 1999). These assumptions match fairly well with the average branching angle and variability recorded in this species (average 58.3 ± 0.75), which may indicate that the branching angle found in the phalanx seagrass Zostera noltii may be an evolutionary trait that would allow to maximise the occupation of the colonized space but reducing the belowground network construction costs. However, in a previous paper (Brun et al 2006a) it was demonstrated that the most efficient colonization pattern in Z. noltii occurred at opposite branching pattern (i.e. first ramet branches in the same direction that the previous one); this is, for instance, the branching pattern recorded in the seagrass Cymodocea nodosa (Brun et al. 2006a and unpublished). Therefore, the pattern of growth and branching angle may be an evolutionary trait in seagrasses, but ecological and inter-specifics consequences are still far to be clarified and they need further attention.
In conclusion, the small sized seagrass Zostera noltii can develop into clones with a considerable number of shoots and internodes. A large fraction of the clone may be clonally integrated to allow feeding in heterogeneous-resource environments and enabling the individual shoots to buffer unfavourable micro-environmental conditions at the whole clone level. Plant architecture seems to be regulated by the interaction between apical dominance strength and clonal integration distance, with clonal behaviour constrained by the clonal growth rules and the growth pattern. The complex relationship between apical dominance and clonal integration in Z. noltii may deserve future research effort to elucidate the seasonal and spatial scale of both processes and the outcome at the plant architectural level. Branching angle did not display any relationship with clonal rules and seemed to be more dependent on the surrounding environmental conditions. Z. noltii seems to maximise occupation of colonization space by branching at angles close to 60°, which reduces the investment in belowground tissues and ramets overlapping.
References
Armstrong RA (1983) On the quatitative theory of resource partitioning in rhizomatous perennil plants: the influence of canopy structure, rhizome branching and self-thinning. Ecology 64:703–709
Bell SS, Fonseca MS, Stafford NB (2006) Seagrass ecology: new contributions from a landscape perspective. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation, vol 625. Springer, The Netherlands, pp 645
Briske DD (1991) Developmental morphology and physiology of grasses. In: Heitschmidt RK, Stuth JW (eds) Grazing management: an ecological perspective. Timber Press, Portland, pp 85–108
Brouns JJWM (1987) Growth patterns in some Indo-West-Pacific seagrasses. Aquat Bot 28:39–61
Brun FG, Pérez-Lloréns JL, Vergara JJ, Hernández I (2003) Patch distribution and within-patch dynamics of the seagrass Zostera noltii Hornem. at Los Toruños salt-marsh (Cádiz Bay, Natural Park, Spain). Bot Mar 46:513–524
Brun FG, Vergara JJ, Hernández I, Pérez-Lloréns JL (2005) Evidence for vertical growth in Zostera noltii Hornem. Bot Mar 48:446–450
Brun FG, Pérez-Pastor A, Hernández I, Vergara JJ, Pérez-Lloréns JL (2006a) Shoot organization in the seagrass Zostera noltii: implications for space occupation and plant architecture. Helg Mar Res 60:59–69
Brun FG, Vergara JJ, Peralta G, García-Sánchez MP, Hernández I, Pérez-Lloréns JL (2006b) Clonal building, simple growth rules and phylloclimate as key steps to develop functional-structural seagrass models. Mar Ecol Prog Ser 323:1–16
Cain ML (1994) Consequences of foraging in clonal species. Ecology 75(4) 993:944
Callaghan TV, Svensson BM, Bowman H, Lindley DK, Carlsson BA (1990) Models of clonal plant growth based on population dynamics and architecture. Oikos 57:257–269
Charpertier A (2002) Consequences of clonal growth for plant mating. Evol Ecol 15:521–530
Cline MG (1996) Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann Bot 78:255–266
Cline MG (1997) Conceps and terminology of apical dominance. Am J Bot 84(9):1064–1069
Coyer JA, Diekmann OE, Serrão EA, Procaccini G, Milchakova N, Pearson GA, Stam WT, Olsen JL (2004a) Population genetics of dwarf eelgrass Zostera noltii throughout its biogeographic range. Mar Ecol Prog Ser 281:51–62
Coyer JA, Reusch TBH, Stam WT, Serrão EA, Pearson GA, Procaccini G, Olsen JL (2004b) Characterization of microsatellite loci in the dwarf eelgrass Zostera noltii (Zosteraceae) and cross-reactivity with Z. japonica. Mol Ecol Notes 4(3):497
De Kroons H, Knops J (1990) Habitat exploration through morphological plasticity in two chalk grassland perennials. Oikos 59:39–59
De kroon H, Schieving F (1991) Resource allocation patterns as a function of clonal morphology: A general model applied to a foraging clonal plant. J Ecol 79(2):519–530
de Kroon H, Huber H, Stuefer JF, van Groenendael JM (2005) A modular concept of phenotypic plasticity in plants. New Phytol 166:73–82
Duarte CM (1991) Allometric scaling of seagrass form and productivity. Mar Ecol Prog Ser 67:201–207
Duarte CM, Fourqurean J, Krause-Jensen D, Olesen B (2006) Dynamics of seagrass stability and change. In: Larkum AWD, Orth RJ and Duarte CM (eds) Seagrasses: biology, ecology and conservation, vol 271. Springer, The Netherlands, pp 294
Groenendael JM, de Kroons H (1990) Clonal growth in plants: regulation and function. SPB Academic Publishing, The Hague, 196 pp. ISBN 90-5103-056-8
Harrison PG (1977) Patterns of uptake and translocation of 14C by Zostera Americana Den Hartog in the laboratory. Aquat Bot 5:93–97
Harrison MA, Kaufman PB (1980). Hormonal regulation of lateral bud (tiller) release in oats (Avena sativa L.). Plant Physiol 66(6):1123–1127
Harrison MA, Kaufman PB (1984) The role of hormone transport and metabolism in apical dominance in oats. Bot Gaz 145:293–297
Hemminga MA (1998) The root/rhizome system of seagrasses: an asset and a burden. J Sea Res 39:183–196
Holm S (1979) A simple sequentially reject multiple test procedure. Scand J Stat 6:65–70
Irwin Dl, Aarssen LW (1996) Effects of nutrient level on cost and benefit of apical dominance in Epilobium ciliatum. Am Midl Nat 136(1):14–28
Kendrick GA, Hegge BJ, Wyllie A, Davidson A, Lord DA (2000) Changes in seagrass cover on Success and Parmelia Banks, Western Australia between 1965 and 1995. Estuar Coast Shelf Sci 50:341–353
Kendrick GA, Duarte Cm, Marbá N (2005) Clonality in seagrasses, emergent properties and seagrass landscape. Mar Ecol Progr Ser 290:291–296
Li Y, Hagen G, Guilfoyle TJ (1992) Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Dev Biol 153:386–395
Libes M, Boudouresque CF (1987) Uptake and long distance transport of carbon in the marine phanerogam Posidonia oceanica. Mar Ecol Prog Ser 38:177–186
Lovett-Doust L (1981) Population dynamics and local specialization in a clonal perennial (Rannunculus repens). The dynamics of ramets in contrasting habitats. J Ecol 69:743–755
Magyar G, Kertész M, Oborny B (2004) Resource trasnport between ramets alters soli resource pattern: a simulation study on clonal plants. Evol Ecol 18:469–492
Marbà N, Duarte CM (1998) Rhizome elongation and seagrass clonal growth. Mar Ecol Prog Ser 174:269–280
Marbà N, Hemminga MA, Mateo MA, Duarte CM, Mass YEM, Terrados J, Gacia E (2002) Carbon and nitrogen translocation between seagrass ramets. Mar Ecol Prog Ser 226:287–300
McIntyre GI (1973) Environmental control of apical dominance on Phaseolis vulgaris. Can J Bot 51:293–299
McIntyre GY (1977) The role of nutrition in apical dominance. Symp Soc Exp Biol 31:251–273
Meyer AH, Schmid B (1999) Experimental demography of rhizome populations of establishing clones of Solidago altissima. J Ecol 87:42–54
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100:403–405
Neuhäuser M (2004) Testing wheter any of the significant tests within a table are indeed significant. Oikos 106:409–410
Niklas KJ (1994) Plant allometry. University of Chicago Press, Chicago, p 412
Oborny B (2004) External and internal control in plant development. Adaptive plasticity in plants. Complexity 9(3):22–28
Oborny B, Czaran T, Kun A (2001) Exploration and exploitation of resource patches by clonal growth: a spatial model on the effect of transport between modules. Ecol Model 141:151–169
Oborny B, Kun A, Czárán T, Bokkos S (2000) The effect of clonal integration on plant competition for mosaic habitat space. Ecology 81(2):3291–3304
Peralta G, Brun FG, Pérez-Lloréns JL, Hernández I, Vergara JJ (2005) Morphometric variations as acclimation mechanisms in the seagrass Zostera noltii: biological effects of coastal engineering works. Est Coast Shelf Sci 64:347–356
Peralta G, Brun FG, Pérez-Lloréns, Bouma TJ (2006) Current velocity directly affects growth, morphometry and architecture of the seagrass Zostera noltii. Mar Ecol Prog Ser (in press)
Phillips IDJ (1969) Apical dominance. In: Williams MB (ed) Physiology and biochemistry of plant growth and development. McGraw-Hill, London, p 163
Phillips IDJ (1975) Apical dominance. Annu Rev Plant Physiol 26:341–367
Prusinkiewicz P (2004) Modelling plant growth and development. Curr Opin Plant Biol 7(1) 79:83
Reusch TBH (2006) Does disturbance enhance genotypic diversity in clonal organisms? A field test in the marine angiosperm Zostera marina. Mol Ecol 15:277–286
Ruggiero MV, Reusch TBH, Procaccini G (2005a) Local genetic structure in a clonal dioecious angiosperm. Mol Ecol 14:4, 957–967
Ruggiero MV, Capone S, Pirozzi P, Reusch TBH, Procaccini G (2005b) Mating system and clonal architecture: a comparative study in two marine angiosperms. Evol Ecol 19:487–499
Schmid BE, Puttick GME, Burgués KHE, Bazzaz FAE (1988) Clonal integration and effects of simulated herbivory in old-field perennials. Oecologia 75(3):465–471
Short FT (1983) The seagrass, Zostera marina L.: plant morphology and bed structure in relation to sediment ammonium in Izembek Lagoon, Alaska. Aquat Bot 16:149–161
Sintes T, Marba N, Duarte CM, Kendrick GA (2005) Nonlinear processes in seagrass colonisation explained by simple clonal growth rules. Oikos 108:165–175
Sintes T, Marbá N, Duarte CM (2006) Modeling nonlinear seagrass clonal growth: assessing the efficiency of space occupation across the seagrass flora. Estuar Coasts 29(1):72–80
Smith Ap, Palmer JO (1976) Vegetative reproduction and close parking in a successional plant species. Nature 261:232–233
Stoll P, Egli P, Schmid B (1998) Plant foraging and rhizome growth patters of Solidago altissima in response to mowing and fertilizer application. J Ecol 86:341–354
Terrados J, Duarte CM, Kenworthy WJ (1997) Experimental evidence for apical dominance in the seagrass Cymodocea nodosa. Mar Ecol Prog Ser 148:263–268
Tomasko DA, Dawes CJ (1989) Evidence for physiological integration between shaded and unshaded short shoots of Thalassia testudinum. Mar Ecol Prog Ser 54(3):299–305
Vidondo B, Duarte CM, Middelboe AL, Stefansen K, Lützen T, Nielsen SL (1997) Dynamics of a landscape mosaic: size and age distributions, growth and demography of seagrass Cymodocea nodosa patches. Mar Ecol Prog Ser 158:131–138
West GB, Brown JH, Enquist BJ (1999) A general model for the structure and allometry of plant vascular systems. Nature 400:664–667
Wilson BF (2000) Apical control of branch and angle in woody plants. Am J Bot 87(5):601–607
Woolley DJ, Wareing PF (1972) The interaction between growth promoters in apical dominance. II. Environmental effects on endogenous cytokinin and gibberellin levels in Solanum andigena. New Phytol 71(6):1015–1025
Zar JH (1984) Biostatistical analysis, 2nd edn. Prentice-Hall, Englewood Cliffs
Zaykin DV, Zhivotovsky LA, Westfall PH, Weir BS (2002) Truncated product method for combining P-values. Genet Epidemiol 22:170–185
Zimmerman RC, Kohrs DG, Steller DL, Alberte RS (1995) Carbon partitioning in eelgrass: regulation by photosynthesis and the response to daily light-dark cycles. Plant Physiol 108:1665–1671
Acknowledgments
FG Brun is supported by an individual Marie Curie fellowship (EU contract MEIF-CT-2005-515071). This work was also funded by the Spanish Projects REN2002-00746/MAR and CTM2005-00395/MAR. I. Olivé holds a FPU grant from the Ministerio de Educación y Ciencia. This is the NIOO publication number 3986.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O Kinne.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Brun, F.G., Cummaudo, F., Olivé, I. et al. Clonal extent, apical dominance and networking features in the phalanx angiosperm Zostera noltii Hornem.. Mar Biol 151, 1917–1927 (2007). https://doi.org/10.1007/s00227-007-0627-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0627-y