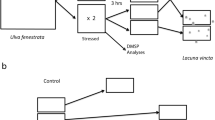
Abstract
Although dimethylsulfoniopropionate (DMSP) has a variety of functions in marine macroalgae including that of a cryoprotectant, an osmolyte, a way to remove excess sulfur and energy, an antioxidant, and an allelopathic precursor, the latter two functions are believed to be the most important in Ulva lactuca L. (=U. fenestrata) in intertidal populations on the coast of Washington state, USA. The present study found significant variation in DMSP concentrations among U. lactuca collected in May 2005 from six sites ranging from 47°54.45′N (Possession Point, Whidbey Island, WA, USA) to 48°30.55′N (Shannon Point Beach, Anacortes, WA, USA), and also among individuals within sites, and among tissues (basal tissues near the holdfast, middle of the blades, and tips). Concentrations ranged from 37 to 224 μmol g−1 fresh mass (FM). In several 10-day experiments between July 2001 and August 2004 with U. lactuca collected from several places on the coast of Washington, the effects of nutrient level (DIN), light intensity and wavelength, and grazing by the herbivorous gastropod Lacuna vincta, were examined. None of these manipulations resulted in DMSP concentrations that differed significantly from controls, and variance in DMSP concentrations within each experiment was very low. Although DMSP concentrations in U. lactuca may be affected by factors not tested in these experiments, it is also possible that the observed spatial differences reflect constitutive genotypic or phenotypic differences among geographically separated U. lactuca populations or among cryptic Ulva species.





Similar content being viewed by others
References
Arnold T, Tanner C, Hatch W (1995) Phenotypic variation in polyphenolic content of the tropical brown alga Lobophora variegata as a function of nitrogen availability. Mar Ecol Prog Ser 123:177–183
Bold H, Wynne M (1985) Introduction to the algae. Prentice-Hall, Englewood Cliffs
Bryant JP, Chapin FSI, Klein DR (1983) Carbon/nutrient balance of boreal plants in relation to herbivory. Oikos 40:357–368
Cantoni G, Anderson D (1956) Enzymatic cleavage of dimethylpropiothetin by Polysiphonia lanosa. J Biol Chem 222:171–177
Cronin G (2003) Resource allocation in seaweeds and marine invertebrates: chemical defense patterns in relation to defense theories. In: McClintock J, Baker BJ (eds) Marine chemical ecology. CRC Press, Boca Raton, pp 325–354
Cronin G, Hay ME (1996a) Chemical defenses, protein content, and susceptibility to herbivory of diploid vs. haploid stages of the isomorphic brown alga Dictyota ciliolata (Phaeophyta). Botanica Marina 39:395–399
Cronin G, Hay ME (1996b) Effects of light and nutrient availability on the growth, secondary chemistry, and resistance to herbivory of two brown seaweeds. Oikos 77:93–106
Denton A, Chapman ARO, Markham J (1990) Size-specific concentrations of phlorotannins (anti-herbivore compounds) in three species of Fucus. Mar Ecol Prog Ser 65:103–104
Edwards DM, Reed RH, Chudek JA, Foster R, Stewart WDP (1987) Organic solute accumulation in osmotically-stressed Enteromorpha intestinalis. Mar Biol 95:583–592
Edwards DM, Reed RH, Stewart WDP (1988) Osmoacclimation in Enteromorpha intestinalis: long-term effects of osmotic stress on organic solute accumulation. Mar Biol 88:457–476
Hay M, Paul V, Lewis S, Gustafson K, Tucker J, Trindell R (1988) Can tropical seaweed to reduce herbivory by growing at night? Diel patterns of growth, nitrogen content, herbivory, and chemical versus morphological defenses. Oecologia 75:233–245
Hayden H, Waaland J (2002) Phylogenetic systematics of the Ulvaceae (Ulvales, Ulvophyceae) using chloroplast and nuclear DNA sequences. J Phycol 28:1200–1212
Hayden H, Waaland J (2004) A molecular systematic study of Ulva (Ulvaceae, Ulvales) from the Northeast Pacific. Phycologia 43:364–382
Hayden H, Blomster J, Maggs C, Silva P, Stanhope M, Waaland J (2003) Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. Eur J Phycol 38:277–294
Ilvessalo H, Tuomi J (1987) Nutrient availability and accumulation of phenolic compounds in the brown alga Fucus vesiculosus. Mar Biol 101:115–119
Karsten U, Wiencke C, Kirst GO (1991) Growth pattern and beta-dimethylsulfoniopropionate (DMSP) content of marine macroalgae at different irradiances. Mar Biol 108:151–155
Karsten U, Wiencke C, Kirst GO (1992) Dimethylsulphonio-propionate (DMSP) accumulation in green macroalgae from polar to temperate regions: interactive effects of light versus salinity and light versus temperature. Polar Biol 12:603–607
Kirst G (1989) Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Physiol Plant Mol Biol 40:21–53
Kirst GO, Thiel C, Wolff H, Nothnagel J, Wanzek M, Ulmke R (1991) Dimethylsulphoniopropionate (DMSP) in ice-algae and its possible biological role. Mar Chem 35:381–388
Newton J, Albertson S, Van Voorhis K, Siegel E (2002) Washington State marine water quality, 1998 through 2000. Washington State Department of Ecology, 02–03–056, Olympia
O’Clair R, Lindstrom S (2000) North Pacific seaweeds. Plant Press, Auke Bay
Padilla DK (1983) Rip stop in marine algae: minimizing the consequences of herbivore damage. Evol Ecol 7:634–644
Paul VJ, Van Alstyne KL (1988) Chemical defense and chemical variation in some tropical Pacific species of Halimeda (Halimediaceae; Chlorophyta). Coral Reefs 6:263–269
Paul VJ, Van Alstyne KL (1992) Activation of chemical defenses in the tropical marine algae Halimeda spp. J Exp Mar Biol Ecol 160:191–203
Puglisi MP, Paul VJ (1997) Intraspecific variation in the red alga Portierria hornemannii: monoterpene concentrations are not influenced by nitrogen or phosphorus enrichment. Mar Biol 128:161–170
Reed RH (1983) The osmotic significance of tertiary sulfonium and quaternary ammonium compounds in marine macroalgae. Br Ecol Soc 18:208
Sieburth JMN (1960) Acrylic acid, and “antibiotic” principle in Phaeocystis blooms in Antarctic waters. Science 132:676–677
Stefels J (2000) Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. J Sea Res 43:183–197
Steinberg PD (1992) Geographical variation in the interaction between marine herbivores and brown algal secondary metabolites. In: Paul V (ed) Ecological roles of marine natural products. Cornell University Press, Ithaca, pp 245
Steinberg PD, Estes JA, Winter FC (1995) Evolutionary consequences of food chain length in kelp forest communities. Proc Nat Acad Sci 92:8145–8148
Sunda WG, Kieber D, Kiene R, Huntsman S (2002) An antioxidant function for DMSP and DMS in marine algae. Nature 418:317–320
Van Alstyne KL (1988) Herbivore grazing increases polyphenolic defenses in the intertidal brown alga Fucus distichus. Ecology 69:655–663
Van Alstyne KL, Houser LT (2003) Dimethylsulfide release during macroinvertebrate grazing and its role as an activated chemical defense. Mar Ecol Prog Ser 250:175–181
Van Alstyne KL, McCarthy JJ III, Hustead CL, Duggins DO (1999) Geographic variation in polyphenolic levels of Northeastern Pacific kelps and rockweeds. Mar Biol 133:371–379
Van Alstyne KL, Ehlig JM, Whitman SL (2000) Ontogenetic shifts in phlorotannin production, nutritional quality, and susceptibility to herbivory in marine brown algae. Mar Biol 180:179–185
Van Alstyne KL, Wolfe GV, Freidenburg TL, Neill A, Hicken C (2001) Activated defense systems in marine macroalgae: evidence for an ecological role for DMSP cleavage. Mar Ecol Prog Ser 213:53–65
Van Alstyne KL, Dethier MN, Duggins DO (2003a) Spatial patterns in macroalgal chemical defenses. In: McClintock J, Baker W (eds) Marine chemical ecology. CRC Press, Boca Raton, pp 301–324
Van Alstyne KL, Pelletreau KN, Rosario K (2003b) The effects of salinity on dimethylsulfoniopropionate production in the green alga Ulva fenestrata Postels et Ruprecht (Chlorophyta). Botanica Marina 46:350–356
Whitledge T, Malloy S, Patton CJ, Wirick C (1981) Automated nutrient analyses in seawater. Brookhaven National Lab, Upton
Wolfe GV, Steinke M, Kirst GO (1997) Grazing-activated chemical defence in a unicellular marine alga. Nature 387:894–897
Wright J, de Nys R, Steinberg P (2000) Geographic variation in halogenated furanones from the red alga Delisea pulchra and associated herbivores and epiphytes. Mar Ecol Prog Ser 207:227–241
Yates JL, Peckol P (1993) Effects of nutrient availability and herbivory on polyphenolics in the seaweed Fucus vesiculosus. Ecology 74:1757–1766
Acknowledgements
We are grateful to H. Ribarich, D. Simunds, and S. Gifford for their help in the field and laboratory, and K. Pelletreau for assisting with the carbon and nitrogen analyses. We also thank Dennis Willows and the staff at the Friday Harbor Marine Laboratory for providing facilities and useful advice. This work was supported by a grant from the Murdock Charitable Trust to T. Nelson (99161:JVX:02/24/00) and National Science Foundation grants to K. Van Alstyne and Shannon Point Marine Center (DBI-9877129, DBI-0090825, and DBI-0098409).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Van Alstyne, K.L., Koellermeier, L. & Nelson, T.A. Spatial variation in dimethylsulfoniopropionate (DMSP) production in Ulva lactuca (Chlorophyta) from the Northeast Pacific. Mar Biol 150, 1127–1135 (2007). https://doi.org/10.1007/s00227-006-0448-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0448-4