Abstract
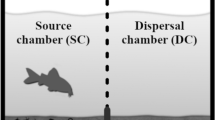
Free-living marine, benthic nematodes quickly colonise sediments where physical forces are strong enough to suspend them into the water column. In the absence of such forces colonisation is much slower and is more likely to be affected by biological factors. The aim of the study was to investigate if nematodes disperse more readily in the presence of biological disturbance where physical disturbance is rare or non-existent. Amphipods are able to greatly rework sediments, and thereby induce disturbance to the infauna. A laboratory experiment with the amphipod Monoporeia affinis and nematodes from a low-energy, 30-m-deep location was conducted in mesocosms where the nematodes were given the choice to colonise azoic sediment at three amphipod densities, zero, low and high. Each area of azoic sediment in the mesocosms was divided into three equilateral sections from the nematode source, i.e. 10, 23 and 36 cm. At termination, after 7 weeks, there were no significant differences in nematode abundance and assemblage structure between treatments despite considerable biological disturbance created by the amphipods. The number of nematodes was 16%, 15% and 11% of the total numbers in the source at the three sections 10, 23 and 36 cm, respectively. There were distinct differences in the nematode community composition between distances, with the small surface-dwelling taxon Leptolaimus spp. being a rapid and the numerically dominant coloniser of the azoic sediments. Migration of nematodes over short distances is likely to be slow in the absence of strong physical forces. To our knowledge, this is the first paper ever that investigates the influence of macrofauna on nematode short-range migration.


Similar content being viewed by others
References
Aarnio K, Bonsdorff E (1992) Colonization rates and community structure of benthic meiofauna in shallow Baltic archipelago waters. Aqua Fenn 22:71–80
Aarnio K, Bonsdorff E, Norkko A (1998) Role of Halicryptus spinulosus (Priapulida) in structuring meiofauna and settling macrofauna. Mar Ecol Prog Ser 163:145–153
Abebe E, Coomans A (1995) Freshwater nematodes of the Galápagos. Hydrobiologia 299:1–51
Albertsson J, Leonardsson K (2000) Impact of a burrowing deposit-feeder, Monoporeia affinis, on viable zooplankton resting eggs in the northern Baltic Sea. Mar Biol 136:611–619
Alongi DM, Boesch DF, Diaz RJ (1983) Colonization of meiobenthos in oil-contaminated subtidal sands in the lower Chesapeake Bay. Mar Biol 72:325–335
Armonies W (1988) Hydrodynamic factors affecting behaviour of intertidal meiobenthos. Ophelia 28:183–193
Austen MC, Widdicombe S (1998) Experimental evidence of effects of the heart urchin Brissopsis lyrifera on associated subtidal meiobenthic nematode communities. J Exp Mar Biol Ecol 222:219–238
Austen MC, Widdicombe S, Villano-Pitacco N (1998) Effects of biological disturbance on diversity and structure of meiobenthic nematode communities. Mar Ecol Prog Ser 174:233–246
Begon M, Harper JL, Townsend CR (1996) Ecology. Blackwell, Oxford
Bell SS (1980) Meiofauna–macrofauna interactions in a high salt marsh habitat. Ecol Monogr 50:487–505
Bertelsen RD (1998) Active and passive settling by marine benthic nematodes. Diss Abstr Int B Sci Eng 58:3425
Billheimer LE, Coull BC (1988) Bioturbation and recolonization of meiobenthos in juvenile spot (Pisces) feeding pits. Estuar Coast Shelf Sci 27:335–340
Bonsdorff E (1992) Drifting algae and zoobenthos—effects on settling and community structure. Neth J Sea Res 30:57–62
Botto F, Iribarne O (1999) Effect of the burrowing crab Chasmagnathus granulata (Dana) on the benthic community of a SW Atlantic coastal lagoon. J Exp Mar Biol Ecol 241:263–284
Chandler GT, Fleeger JW (1983) Meiofaunal colonization of azoic estuarine sediment in Louisiana: mechanisms of dispersal. J Exp Mar Biol Ecol 69:175–188
Commito JA, Tita G (2002) Differential dispersal rates in an intertidal meiofauna assemblage. J Exp Mar Biol Ecol 268:237–256
Crofton HD (1966) Nematodes. Hutchinson University Library, London
De Jonge VN, Bouwman LA (1977) A simple density separation technique for quantitative isolation of meiobenthos using the colloidal Silica Ludox-TM. Mar Biol 42:143–148
DePatra KD, Levin LA (1989) Evidence of the passive deposition of meiofauna into fiddler crab burrows. J Exp Mar Biol Ecol 125:173–192
Dobbs FC, Guckert JB (1988) Callianassa trilobata (Crustacea: Thalassinidea) influences abundance of meiofauna and biomass, composition, and physiologic state of microbial communities within its burrow. Mar Ecol Prog Ser 45:69–79
Fegley SR (1985) Experimental studies on the erosion of meiofauna from soft-substrates by currents and waves. Diss Abstr Int B Sci Eng 46:174
Fleeger JW, Chandler GT, Fitzhugh GR, Phillips FE (1984) Effects of tidal currents on meiofauna densities in vegetated salt marsh sediments. Mar Ecol Prog Ser 19:49–53
Grant WD, Williams III AJ, Glenn SM (1984) Bottom stress estimates and their prediction on the northern California continental shelf during CODE-1: the importance of wave–current interaction. J Phys Oceanogr 14:506–527
Gray J, Lissman HW (1964) The locomotion of nematodes. J Exp Biol 41:135–154
Hagerman Jr GM, Rieger RM (1981) Dispersal of benthic meiofauna by wave and current action in Bogue Sound, North Carolina, USA. Mar Ecol 2:245–270
Heip C, Vincx M, Vranken G (1985) The ecology of marine nematodes. Oceanogr Mar Biol Annu Rev 23:399–489
Hendelberg M, Jensen P (1993) Vertical distribution of the nematode fauna in a coastal sediment influenced by seasonal hypoxia in the bottom water. Ophelia 37:83–94
Hill C, Elmgren R (1987) Vertical distribution in the sediment in the co-occurring benthic amphipods Pontoporeia affinis and P. femorata. Oikos 49:221–229
Hurlbert SH (1984) Pseudoreplication and the design of ecological experiments. Ecol Monogr 54:187–211
Jacobs LJ (1984) The free-living inland aquatic nematodes of Africa—a review. Hydrobiologia 113:259–291
Jensen P (1981) Phyto-chemical sensitivity and swimming behaviour of the free-living marine nematode Chromadorita tenuis. Mar Ecol Prog Ser 4:203–206
Kachigan SK (1991) Multivariate statistical analysis: a conceptual introduction. Radius Press, New York
Kern JC, Taghon GL (1986) Can passive recruitment explain harpacticoid copepod distributions in relation to epibenthic structure? J Exp Mar Biol Ecol 101:1–23
Köhn J (1995) Amphipods of the Baltic Sea. Pol Arch Hydrobiol 42:385–394
Kramer CY (1956) Extension of multiple range tests to group means with unequal numbers of replications. Biometrics 12:307–310
Kruskal JB (1964) Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29:1–27
Lindström M, Lindström A (1980) Swimming activity of Pontoporeia affinis (Crustacea, Amphipoda)—Seasonal variations and usefulness for environmental studies. Ann Zool Fenn 17:213–220
Milbrink G, Timm T (2001) Distribution and dispersal capacity of the Ponto-Caspian tubificid oligochaete Potamothrix moldaviensis Vejdovsky et Mrazek, 1903 in the Baltic Sea region. Hydrobiologia 463:93–102
Mott JB, Harrison AD (1983) Nematodes from river drift and surface drinking water supplies in southern Ontario. Hydrobiologia 102:27–38
Ólafsson E, Elmgren R (1991) Effects of biological disturbance by benthic amphipods Monoporeia affinis on meiobenthic community structure: a laboratory approach. Mar Ecol Prog Ser 74:99–107
Ólafsson E, Elmgren R (1997) Seasonal dynamics of sublittoral meiobenthos in relation to phytoplankton sedimentation in the Baltic Sea. Estuar Coast Shelf Sci 45:149–164
Ólafsson E, Moore CG (1990) Control of meiobenthic abundance by macroepifauna in a subtidal muddy habitat. Mar Ecol Prog Ser 65:241–249
Ólafsson E, Moore CG (1992) Effects of macroepifauna on developing nematode and harpacticoid assemblages in a subtidal muddy habitat. Mar Ecol Prog Ser 84:161–171
Ólafsson E, Elmgren R, Papakosta O (1993) Effects of the deposit-feeding benthic bivalve Macoma baltica on meiobenthos. Oecologia 93:457–462
Palmer MA (1983) The role of behavior and flow in the dispersal of marine meiofauna. Dissertation, Marine Science Program, University of South Carolina, Columbia
Palmer MA, Gust G (1985) Dispersal of meiofauna in a turbulent tidal creek. J Mar Res 43:179–210
Patterson RT, McKillop WB, Kroker S, Nielsen E, Reinhardt EG (1997) Evidence for rapid avian-mediated foraminiferal colonization of Lake Winnipegosis, Manitoba, during the Holocene Hypsithermal. J Paleolimnol 18:131–143
Peters BG (1928) On the bionomics of the vinegar eelworm. J Helminthol 6:1–38
Platt HM, Warwick RM (1980) The significance of free-living nematodes to the littoral ecosystem. In: Price J, Irvine D, Farnham W (eds) The shore environment, vol 2: ecosystems. Academic, London, pp 729–759
Platt HM, Warwick RM (1983) Free-living marine nematodes, part 1: British enoplids: pictorial key by world genera and notes for the identification of British species. Cambridge University Press, Cambridge
Platt HM, Warwick RM (1988) Free-living marine nematodes, part 2. British chromadorids: pictorial key to world genera and notes for the identification of British species. BrillBackhuys, Leiden
Powers SP (1998) Recruitment of soft-bottom benthos (benthic invertebrates, encrusting community, infaunal community). Diss Abstr Int B Sci Eng 58:5760
Reise K (1979) Moderate predation on meiofauna by the macrobenthos of the Wadden Sea. Helgol Wiss Meeresunters 32:453–465
Savidge WB, Taghon GL (1988) Passive and active components of colonization following two types of disturbance on intertidal sandflat. J Exp Mar Biol Ecol 115:137–155
Schneider G (1914) Nematoden als fischnahrung. Int Rev Gesamten Hydrobiol Hydrogr 6:489–490
Schratzberger M, Rees HL, Boyd SE (2000a) Effects of simulated deposition of dredged material on structure of nematode assemblages—the role of burial. Mar Biol 136:519–530
Schratzberger M, Rees HL, Boyd SE (2000b) Effects of simulated deposition of dredged material on structure of nematode assemblages—the role of contamination. Mar Biol 137:613–622
Segerstråle SG (1957) Baltic Sea. Geol Soc Am Mem 67:757–800
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Sherman KM, Coull BC (1980) The response of meiofauna to sediment disturbance. J Exp Mar Biol Ecol 46:59–71
Sherman KM, Reidenauer JA, Thistle D, Meeter D (1983) Role of natural disturbance in an assemblage of marine free-living nematodes. Mar Ecol Prog Ser 11:23–30
Sibert JR (1981) Intertidal hyperbenthic populations in the Nanaimo Estuary. Mar Biol 64:259–265
Soetaert K, Van Rijswijk P (1993) Spatial and temporal patterns of the zooplankton in the Westerschelde estuary. Mar Ecol Prog Ser 97:47–59
Surey-Gent SC (1981) Distribution of Anoplostoma viviparum (Nematoda) in Southampton water sediments. Mar Biol 62:157–160
Thistle D (1980) The response of a harpacticoid copepod community to a small scale natural disturbance. J Mar Res 38:381–395
Tita G, Desrosiers G, Vincx M, Nozais C (2000) Predation and sediment disturbance effects of the intertidal polychaete Nereis virens (Sars) on associated meiofaunal assemblages. J Exp Mar Biol Ecol 243:261–282
Tukey JW (1994) The problem of multiple comparisons. Previously unpublished manuscript (1953). In: Braun HI, Kaplan B, Sheehan KM, Wang M-H (eds) The collected works of John W. Tukey, vol VIII. Multiple comparisons: 1948–1983. Chapman and Hall, New York, pp 1–300
Vriser B (1998) Meiofaunal recolonization of defaunated sediments: a field experiment; preliminary results. Period Biol 100:63–69
Wallace HR, Doncaster CC (1964) A comparative study of the movement of some microphagous, plant–parasitic and animal–parasitic nematodes. Parasitology 54:313–326
Warwick RM, Clarke KR, Gee JM (1990a) The effect of disturbance by soldier crabs Mictyris platycheles H. Milne Edwards on meiobenthic community structure. J Exp Mar Biol Ecol 135:19–33
Warwick RM, Clarke KR, Suharsono (1990b) A statistical analysis of coral community responses to the 1982–83 El Niño in the Thousand Islands, Indonesia. Coral Reefs 8:171–179
Warwick RM, McEvoy AJ, Thrush SF (1997) The influence of Atrina zelandica Gray on meiobenthic nematode diversity and community structure. J Exp Mar Biol Ecol 214:231–247
Widbom B (1983) Colonization of azoic sediment by sublittoral meiofauna in Gullmar Fjord—Swedish West Coast. Oceanol Acta Spec vol:213–217
Acknowledgements
Thanks to the staff at Askö field station, Trosa archipelago. G. Malmberg provided indispensable help with nematode measurements in the image analysis laboratory, and K. Ullberg helped to improve the language. We also thank two referees for useful comments that aided in improving the manuscript. We also acknowledge financial support from Helge Ax:son Johnsons Stiftelse to J.U.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Hagerman, Helsingør
Rights and permissions
About this article
Cite this article
Ullberg, J., Ólafsson, E. Effects of biological disturbance by Monoporeia affinis (Amphipoda) on small-scale migration of marine nematodes in low-energy soft sediments. Marine Biology 143, 867–874 (2003). https://doi.org/10.1007/s00227-003-1139-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1139-z