Abstract
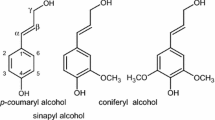
This study reports on the isolation of bamboo lignophenols (LPs) and alkaline lignins (ALs) followed by their subsequent modification by methylolation. Methylolated lignins are quite useful and in high demand in wood–plastics composites or phenolic resin. The structural characterizations of the two lignin samples both before and after methylolation were compared by gel permeation chromatography, Fourier transform infrared spectroscopy, and 1H–13C heteronuclear single quantum coherence nuclear magnetic resonance spectroscopy (2D HSQC NMR). It was found that after methylolation, the weight average molecular weight (Mw) and polydispersity (D = Mw/Mn) of the LPs varied to a greater extent than those of the ALs (c.f., Mw: from 1167 (D = 1.96) to 8267 (D = 5.47) for the LPs and Mw: from 917 (D = 1.45) to 1580 (D = 1.62) for the ALs). These results indicated that the LPs have more functional groups prone to methylolation compared to ALs. Quantitative 13C and 2D-HSQC NMR analysis demonstrated that both LPs and ALs were hydroxyl phenol, guaiacyl, syringyl-type (HGS-type), and that the esterified p-coumarate groups in the native bamboo lignins were cleaved under alkaline cooking conditions but were not cleaved in the phase separation system. In addition, the 1,1-bis(aryl)propane-2-O-aryl ether unit was confirmed to be the basic unit of the LPs, and both the introduced and unreacted p-cresol moieties were observed by 2D HSQC NMR spectroscopy. Finally, the higher ratio of syringyl unit to guaiacyl unit (S/G ratio) and methoxy group content of the ALs compared to the LPs explained the poorer reactivity of the ALs toward methylolation.








Similar content being viewed by others
References
Calvo-Flores FG, Dobado JA (2010) Lignin as renewable raw material. ChemSusChem 3:1227–1235
Dai X (2016) Enhancement and surface hydrophobization on pulp fiber sheet by using lignophenols. Dissertation, Nanjing Forestry University
Dai X, Qian S, Ren H, Omori S (2015) Characterization and application of bamboo (Sinocalamus affinis) lignophenols in lignophenols-pulp sheet composites. BioResources 10(2):3146–3153
del Rio JC, Rencoret J, Prinsen P, Martinez AT, Ralph J, Gutierrez A (2012) Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J Agric Food Chem 60:5922–5935
Funaoka M (1998) A new type of phenolic lignin-based network polymer with the structure-variable function composed of 1,1-diarylpropane units. Polym Int 47:277–290
Funaoka M (2013) Sequential transformation and utilization of natural network polymer “Lignin”. React Funct Polym 73:396–404
Funaoka M, Abe I (1989) Rapid separation of wood into carbohydrate and lignin with concentrated acid-phenol system. Tappi 72(8):145–149
Kim H, Ralph J, Akiyama T (2008) Solution-state 2D NMR of ball-milled plant cell wall gels. Bioenergy Res 1:56–66
Kuo M, Hse CY, Huang DH (1991) Alkali treated lignin as a component in flakeboard resin. Holzforschung 45:47–54
Lan W, Lu FC, Regner M, Zhu YM, Rencorret J, Ralph SA, Zakai UI, Morreel K, Boerjan W, Ralph J (2015) Tricin, a flavonoid monomer in monocot lignification. Plant Physiol 167:1284–1295
Lora JH, Glasser WG (2002) Recent industrial applications of lignin: a sustainable alternative to non-renewable materials. J Polym Environ 10:39–48
Mikame K, Funaoka M (2006) Polymer structure of lignophenol I structure and function of fractionated lignophenol. Polym J 38(6):585–591
NREL/TP-510-42618 (2012) Determination of structural carbohydrates and lignin in biomass. National Renewable Energy Laboratory, Golden
Ren H, Funaoka M (2007) Function and potential of bamboo lignins. Trans Mater Res Soc Jpn 32(4):1095–1098
Rencoret J, Prinsen P, Gutierrez A, Martinez AT, del Rio JC (2015) Isolation and structural characterization of the milled wood lignin, dioxane lignin, and cellulolytic lignin preparations from brewer’s spent grain. J Agric Food Chem 63:603–613
Shimizu S, Yokoyama T, Matsumoto Y (2015) Effect of type of aromatic nucleus in lignin on the rate of the β–O–4 bond cleavage during alkaline pulping process. J Wood Sci 61:529–536
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83(1):1–11
TAPPI T244 CM-1999 (1999) Acid-insoluble ash in wood, pulp, paper and paperboard. Chemical Properties Committee of the Process and Product Quality Division
Vázquez G, González S, Freire S (1997) Effect of chemical modification of lignin on the gluebond performance of lignin-phenolic resins. Bioresour Technol 60:191–198
Wen JL, Sun ZJ, Sun RC (2010) Structural characterization of alkali-extractable lignin fractions from bamboo. Biobased Mater Bioenergy 4:408–425
Wen JL, Xue BL, Xu F (2012) Unveiling the structural heterogeneity of bamboo lignin by in situ HSQC NMR technique. Bioenergy Res 5:886–903
Wen JL, Sun SL, Xue BL, Sun RC (2013a) Recent advances in characterization of lignin polymer by solution-state nuclear magnetic resonance (NMR) methodology. Materials 6:359–391
Wen JL, Xue BL, Xu F, Sun RC, Pinkert A (2013b) Unmasking the structural features and property of lignin from bamboo. Ind Crops Prod 42:332–343
Wen JL, Sun SL, Xue BL, Sun RC (2013c) Quantitative structural characterization of the lignins from the stem and pith of bamboo (Phyllostachys pubescens). Holzforschung 67(6):613–627
Zhang LM, Gellerstedt G (2007) Quantitative 2D HSQC NMR determination of polymer structures by selecting suitable internal standard references. Magn Reson Chem 45:37–45
Acknowledgements
The authors are grateful to the National Key Basic Research Program of China (Project No. 2017YFD0601005), and the National Natural Science Foundation of China (Project No. 31470599). The work was also supported by the Nanjing Forestry University Outstanding Youth Fund (NLJQ2015-5) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, H., Zhu, L. Comparison of the structural characterization of lignophenols and alkaline lignins before and after methylolation. Wood Sci Technol 52, 1133–1151 (2018). https://doi.org/10.1007/s00226-018-1010-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-018-1010-5