Abstract
The epidemiology and pathogenesis of fractures in postmenopausal women has previously been investigated in the Global Longitudinal study of Osteoporosis in Women (GLOW). To date, however, relationships between bone imaging outcomes and fracture have not been studied in this cohort. We examined relationships between high-resolution peripheral quantitative computed tomography (HRpQCT) parameters and fracture in the UK arm of GLOW, performing a cluster analysis to assess if our findings were similar to observations reported from older participants of the Hertfordshire Cohort Study (HCS), and extended the analysis to include tibial measurements. We recorded fracture events and performed HRpQCT of the distal radius and tibia and dual-energy X-ray absorptiometry (DXA) of the hip in 321 women, mean age 70.6 (SD 5.4) years, identifying four clusters at each site. We saw differing relationships at the radius and tibia. Two radial clusters (3 and 4) had a significantly lower hip areal bone mineral density (p < 0.001) compared to Cluster 1; only individuals in Cluster 4 had a significantly higher risk of fracture (p = 0.005). At the tibia, clusters 1, 3 and 4 had lower hip areal bone mineral density (p < 0.001) compared to Cluster 2; individuals in Cluster 3 had a significantly higher risk of fracture (p = 0.009). In GLOW our findings at the radius were very similar to those previously reported in the HCS, suggesting that combining variables derived from HRpQCT may give useful information regarding fracture risk in populations where this modality is available. Further data relating to tibial HRpQCT-phenotype and fractures are provided in this paper, and would benefit from validation in other studies. Differences observed may reflect age differences in the two cohorts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a disease characterised by loss of bone mass and structural deterioration, resulting in increased bone fragility and propensity to fracture. It is a major public health problem, with a high impact on quality of life and high rates of morbidity. Worldwide, there are nearly nine million osteoporotic fractures each year [1]. The burden of fragility fractures will grow with ageing of the population; the US Surgeon General’s report of 2004, consistent with data from the UK, suggested that almost one in two women and one in five men will experience a fracture in their remaining lifetime from the age of 50 years [2]. The economic cost of osteoporosis and fractures are projected to increase in the EU from €37.4 billion in 2010 to €46.8 billion by 2025 and, in the US, from $17 billion in 2005 to $25.3 billion by 2025 [3, 4].
In clinical practice, the definition of osteoporosis relies on measurements of areal bone mineral density (aBMD) by dual-energy X-ray absorptiometry (DXA) [5]. While aBMD is a significant predictor of fracture risk, it is limited because of its two-dimensional nature, which is affected by the size and position of the subject and cannot distinguish between cortical and trabecular compartments. Epidemiological data indicate that a significant proportion of fractures occur in women who would not be classified as osteoporotic according to current aBMD criteria, highlighting the limitations of this approach and the need for other assessment methods to determine underlying causes of bone fragility [6, 7]. Recent advances in imaging permit the assessment of bone microstructure in vivo using high-resolution peripheral quantitative computed tomography (HRpQCT). This imaging modality has been utilized in research settings to examine factors, including skeletal properties of cortical bone and trabecular microarchitecture, that may contribute to fracture risk [8,9,10,11,12].
So far, most studies investigating aetiology of fracture have analysed specific components of bone structure assessing differences in single outcomes between fracture and non-fractured cases [8,9,10,11]. However, cluster analysis allows us to use the data derived from such scans to define bone phenotypes taking into account all parameters derived from HRpQCT scans. A recent study of older men and women, however, demonstrated that two separate phenotypes were associated with high fracture rates, using such mathematical cluster analysis of bone size, volumetric density (vBMD) and microarchitecture from HRpQCT [12]. In the first phenotype, cortical parameters differed with mean cortical thickness and cortical vBMD lower than the sample mean, whereas the second phenotype was characterised by deficiencies in predominantly trabecular bone with lower values than the sample mean. Replication of these findings in an unrelated cohort was a key conclusion of this study and was the rationale of undertaking this current work in the Global Longitudinal study of Osteoporosis in Women (GLOW) study. The epidemiology and pathogenesis of fractures in postmenopausal women has been widely investigated in GLOW—a prospective, multinational, observational, population-based study of postmenopausal women who were 55 years of age and older [13,14,15,16,17,18,19,20]. However, relationships between bone imaging outcomes and fracture rates have not previously been examined in this cohort. Women who participated in the UK component of the GLOW underwent DXA and HRpQCT of the distal radius and tibia. Extensive phenotyping of HRpQCT images allowed the assessment of relationships between individual HRpQCT parameters and fracture, and a cluster analysis which we undertook to assess if the findings were similar to observations reported in older participants of the Hertfordshire Cohort Study, and extended to the tibial site.
Materials and Methods
Study Participants
GLOW is a prospective, observational cohort study conducted through general physician practices in 10 countries. Study design and recruitment have been described in detail previously [21]. In brief, practices, representative of each region, were recruited through primary care networks and provided the names of women aged 55 years and older who had been seen by their physician in the past 24 months. The primary aim of GLOW was to characterise the descriptive epidemiology and health impact of osteoporosis-related fractures among women who were 55 years of age and older worldwide. Globally, GLOW enrolled over 60,000 women through over 700 physicians in 10 countries, and conducted annual follow-up for up to 5 years through annual patient questionnaires. In Southampton only, participants with baseline data and at least one follow-up questionnaire were invited, after completion of 5 years of follow-up, for a follow-up study which included DXA and HRpQCT. Participants were scanned between April 2014 and September 2016. Patients, who were institutionalized or were not able to complete the study survey by themselves due to cognitive impairment, language barriers, institutionalization, or were too ill to complete the survey or attend for the scans were excluded.
Questionnaires
Information was collected using self-administered questionnaires and included details regarding smoking status, alcohol consumption, education level, use of anti-osteoporotic medication (AOM), years since menopause and use of oestrogen or hormone replacement therapy (HRT). Participants were also asked to rate how physically active they were compared to other women of the same age out of the following possible responses: ‘very active’, ‘somewhat active’, ‘a little’ and ‘not at all’. Subjects were considered to be taking anti-osteoporosis medication if, from baseline to the 5-year follow-up, they reported current use of alendronate, calcitonin, etidronate, ibandronate, pamidronate, raloxifene, risedronate, strontium ranelate, teriparatide, tibolone or zoledronic acid. Fracture history was ascertained at baseline and further information on fractures was obtained after 1-, 2-, 3- and 5-year follow-up. Fracture location included the following: clavicle, upper arm, wrist, spine, rib, hip, pelvis, ankle, upper leg and lower leg. Fractures that were reported at baseline, or accrued over 5 years of follow-up were included; hence the fractured subjects were those with prevalent fracture at the time of scan.
Anthropometry and Dual-Energy X-ray Absorptiometry (DXA)
Height was measured to the nearest 0.1 cm using a Marsden stadiometer; weight was measured to the nearest 0.1 kg on the day of scanning using a Marsden MPPS-250 (Marsden Weighing Machine Group Limited, Rotherham, UK) digital floor scale.
Total hip areal bone mineral density (aBMD, g/cm2) was measured at both sides using DXA Hologic Horizon W; software version Apex 5.5.3.1 (Vertec Scientific, Reading, UK); the total effective dose equivalent of the hip scans was 4.7 microsieverts.
Assessment of Bone by HRpQCT
Each participant underwent a HRpQCT scan of the non-dominant distal radius and tibia using XtremeCT I, (Scanco Medical, Basserdorf, Switzerland); if there was a history of fracture on the non-dominant limb, the non-fractured limb was measured. A stack of 110 parallel HRpQCT slices were acquired with an isotropic voxel size of 82 µm. Methods used to process the HRpQCT data have been described previously [9]. The standard evaluation and cortical porosity scripts were run to obtain estimates of total area, trabecular area, cortical area, cortical volumetric density, trabecular volumetric density, trabecular number, trabecular thickness, trabecular separation, cortical porosity and cortical thickness [22]. Of participants with radius scans, 93 of 442 participants had grade 5 scans and were excluded; of participants with tibial scans, 15 of 447 had grade 5 scans and were excluded. The main analysis sample consisted of 321 individuals with complete data on fracture history and the radial HRpQCT parameters; analysis of the tibial HRpQCT parameters was based on a subset of 306/321 participants who also had complete data on the tibial HRpQCT parameters.
Statistical Analysis
Linear regression was used to examine the relationships between individual HRpQCT parameters and fracture history. Unadjusted and fully adjusted associations, accounting for age at time of HRpQCT scan, height, BMI, physical activity, smoking status, alcohol consumption, education, time since last period, use of AOM and oestrogen/HRT, were examined.
The k-means partitioning method of cluster analysis was used to produce clusters of the HRpQCT parameters for the tibia and radius separately. The number of clusters selected was based on the stability of the clustering, and on the potential for identifying contrasting phenotypes (12). The means and standard deviations (SD) of the standardized HRpQCT parameters, and fracture proportion were then determined for each cluster. Poisson regression with robust variance estimation was used to determine the likelihood of fracture in each cluster compared to the lowest risk cluster. Mean total hip aBMD in each cluster was compared to the cluster with the lowest fracture risk. Data were analysed using Stata, version 14.0.
Results
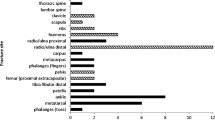
The characteristics of the study population are shown in Table 1. The mean (SD) age of the 321 participants studied was 70.6 (5.4) years at the time of the radius scan. Overall, 63 (19.6%) women reported a fracture among at least one of the fracture locations. The most common fracture site was at the wrist with 25 fractures (32.5% of all fractures among the 10 fracture locations), followed by ankle [15 fractures], rib [11], lower leg [11], upper arm [6], spine [4], hip [2], clavicle [2], pelvis [1] and upper leg [0]. Less than 6% of women were smokers; and a vast majority (91%) did not exceed the recommended limits of alcohol intake.
HRpQCT Parameters and Fracture Status
The associations between fracture history and individual radius and tibia HRpQCT parameters are presented in Table 2. History of fracture was associated with lower radial cortical porosity (p = 0.012), trabecular density (p = 0.001) and trabecular number (p < 0.001), and higher trabecular separation (p < 0.001). These associations were robust to adjustment. At the tibia, history of fracture was associated with lower trabecular density (p = 0.002) and number (p < 0.001), and higher trabecular separation (p < 0.001); associations regarding trabecular number and trabecular separation were robust to adjustment.
Cluster Analysis of Radial HRpQCT Parameters
Four clusters were obtained. The summary statistics of the standardized HRpQCT parameters, hip aBMD and fracture prevalence according to the different clusters are illustrated in Table 3 and Fig. 1.
Means of standardized radial HRpQCT parameters according to each cluster. HRpQCT radial parameters included the following: total area, trabecular area, cortical area, cortical thickness, cortical density, cortical volumetric density, cortical porosity, trabecular volumetric density, trabecular number, trabecular thickness and trabecular separation
In Cluster 4, there was a trend towards lower trabecular density and number and higher trabecular separation compared to the analysis sample (differences in means >0.9 SDs). In this cluster, hip aBMD was significantly lower (p < 0.001) and individuals had a significantly higher risk of fracture [relative risk (95% CI) compared to Cluster 1: 2.49 (1.32, 4.67), p = 0.005]. In contrast to a trabecular deficiency pattern in Cluster 4, Cluster 3 showed differences predominantly in cortical parameters with trend towards lower cortical area, cortical thickness and cortical density, and higher trabecular area compared to the measured sample (differences in means exceeded one SD). Hip aBMD in this cluster was significantly lower, but there was no significant difference in fracture risk compared to Cluster 1.
Similarly in Cluster 1, differences were predominantly in cortical parameters, but here with a trend towards higher cortical area, cortical thickness and cortical density compared to the measured sample (differences in means >0.95 SDs). As expected, total hip aBMD was the highest and fracture risk was the lowest in this cluster.
Cluster 2 had higher trabecular density and lower trabecular separation, but there were no other HRpQCT parameter with means that differed by more than one SD compared to the sample mean. There was no significant difference in hip aBMD or fracture risk in this cluster. Adjustment for hip aBMD throughout did not remove previously observed associations, except that the associations for trabecular density of the radius were attenuated when additionally adjusted for aBMD.
Cluster Analysis of Tibial HRpQCT Parameters
Four clusters were obtained among the 306 participants with complete data for the tibia parameters. The summary statistics of the standardized HRpQCT parameters, hip aBMD and fracture prevalence according to the different clusters are illustrated in Table 4.
Fracture risk was lowest and hip aBMD was highest in Cluster 2. This cluster had lower trabecular area and higher cortical area, thickness and density compared to the analysis sample (differences in means exceeded one SD). Cluster 3 had the highest risk of fracture and the lowest hip aBMD; this cluster was characterised by higher total and trabecular area and lower trabecular density compared to the analysis sample. For the other clusters, none of the tibia parameters differed from the analysis sample by more than one SD.
Discussion
This study demonstrated that microstructural parameters of the bone evaluated by HRpQCT are different between healthy participants and fracture participants at skeletal regions containing predominantly trabecular bone. Trabecular parameters assessed by HRpQCT provided additional skeletal information to that captured from the standard areal bone mineral density (BMD) measurements by DXA. A cluster analysis of the radial and tibial HRpQCT parameters derived one cluster with a significantly higher fracture risk. Individuals in this cluster had lower trabecular density and number, and consequently higher trabecular separation compared to the wider sample. In this cluster, hip aBMD was significantly lower.
An aim of this study was to attempt to replicate findings from the Hertfordshire Cohort Study [12]. We showed that various indices of bone microarchitecture of the radius, most notably cortical porosity, trabecular density, trabecular number and trabecular separation, appeared to be compromised among postmenopausal UK women with a previous history of fracture. These results are in agreement with findings from Hertfordshire [12] and another published study [11] suggesting that alterations of trabecular architecture are likely to play an important role in skeletal fragility associated with osteoporosis [21]. In this study, the results for trabecular parameters described above remained robust to adjustments for demographic and lifestyle factors indicating that results are not due to confounding. Interestingly, and perhaps unexpectedly, history of fracture was associated with lower cortical porosity. Fracture cases had higher cortical area, consistent with findings from other cohorts, however, they also had higher cortical vBMD which is probably due to the lower porosity. This observation has now been made in both the Hertfordshire and GLOW cohorts, and warrants further investigation.
We did see differences in relationships at the radius and tibia which require validation in other samples. This may reflect technical differences in acquisition at the two sites, or differences due to the weight bearing/non-weight bearing nature of the two sites. Fractures in this group were more typically reported at the distal radius, which may also be relevant.
Cluster analysis of the radial HRpQCT parameters demonstrated one phenotype associated with higher risks of fracture. The altered parameters in this cluster included lower trabecular density and number and higher trabecular separation. This is consistent with the previous study on cluster analysis of bone microarchitecture from HRpQCT and fracture risk [12]. Similarly hip aBMD was low in this cluster when compared to the reference cluster in both studies. Interestingly, there was one more very similar phenotype derived by cluster analysis in both studies. It was characterised by higher trabecular area and lower cortical area, thickness and density. In this study, this cluster was not associated with higher fracture risk which is in contrast to the previously published study, where the participants were recruited from the Hertfordshire Cohort Study (HCS). Participants of the HCS were older, of mean age with and without a fracture 77.2 (2.4) and 76.0 (2.6), respectively, compared to participants in our study [mean age of 70.6 (5.4) at time of scan]. In the HCS, there were also differences in phenotype between genders where one cluster associated with high rates of fracture was characterised by low cortical thickness and density in men and women, but in men only, a cluster characterised by higher total and trabecular area was associated with increased fracture risk. Moreover, this cluster in men was not associated with low femoral neck areal BMD. In GLOW, only females were recruited but higher trabecular and total area (in addition to lower trabecular density) were the characteristics of Cluster 3 significantly associated with fracture risk, suggesting a consistency of phenotype.
This study has some limitations. As it is a cross-sectional study, causality cannot be determined since it is not possible to know whether bone microarchitecture changes preceded the fracture. Well-designed prospective studies providing longitudinal data are therefore very important. Although it is reported that cluster analysis models can be very unstable, which could affect the generalizability of the findings in this study, the results were largely consistent to a study by Edwards et al. [12].
In conclusion, this study indicates a phenotype with a significantly higher fracture risk, using cluster analysis of radial and tibial HRpQCT parameters. This approach may have clinical utility in patients where such scans are available, as it allows the incorporation of a large number of variables acquired during a scan to be combined into a bone phenotype that may be more useful for a clinician and patient alike. While our observations were generally in accord with those found in the Hertfordshire Cohort Study, we did note some differences that may reflect the demographic differences between the two groups, particularly age. Given the number of cohorts where HRpQCT data are available, we would welcome attempts at similar analyses. Ultimately our study adds to the growing body of evidence demonstrating distinct phenotypes of bone fragility, which may have implications for targeted prevention and treatment of osteoporosis in the future. Further research is required to examine the identified phenotype and its ability to predict future fracture.
References
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
van Staa TP, Dennison EM, Leufkens HG, Cooper C (2001) Epidemiology of fractures in England and Wales. Bone 29:517–522
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 22:465–475
Kanis JA, Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos Int 4:368–381
Schuit SCE, van der Klift M, Weel AEAM, de Laet CEDH, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JPTM, Pols HAP (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34:195–202
Premaor MO, Pilbrow L, Tonkin C, Parker RA, Compston J (2010) Obesity and fractures in postmenopausal women. J Bone Miner Res 25:292–297
Stein EM, Liu XS, Nickolas TL et al (2010) Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res 25:2572–2581
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90:6508–6515
Ostertag A, Collet C, Chappard C, Fernandez S, Vicaut E, Cohen-Solal M, de Vernejoul MC (2013) A case-control study of fractures in men with idiopathic osteoporosis: fractures are associated with older age and low cortical bone density. Bone 52:48–55
Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD (2007) Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res 22:425–433
Edwards MH, Robinson DE, Ward KA, Javaid MK, Walker-Bone K, Cooper C, Dennison EM (2016) Cluster analysis of bone microarchitecture from high resolution peripheral quantitative computed tomography demonstrates two separate phenotypes associated with high fracture risk in men and women. Bone 88:131–137
Compston JE, Watts NB, Chapurlat R et al (2011) Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med 124:1043–1050
Pfeilschifter J, Cooper C, Watts NB et al (2012) Regional and age-related variations in the proportions of hip fractures and major fractures among postmenopausal women: the Global Longitudinal Study of Osteoporosis in Women. Osteoporos Int 23:2179–2188
Costa AG, Wyman A, Siris ES et al (2013) When, where and how osteoporosis-associated fractures occur: an analysis from the Global Longitudinal Study of Osteoporosis in Women (GLOW). PLoS ONE 8:e83306
Siris ES, Gehlbach S, Adachi JD et al (2011) Failure to perceive increased risk of fracture in women 55 years and older: the Global Longitudinal Study of Osteoporosis in Women (GLOW). Osteoporos Int 22:27–35
Sambrook PN, Flahive J, Hooven FH et al (2011) Predicting fractures in an international cohort using risk factor algorithms, without bone mineral density. J Bone Miner Res 26:2770–2777
Díez-Pérez A, Adachi JD, Adami S et al (2014) Risk factors for treatment failure with antiosteoporosis medication: the Global Longitudinal Study of Osteoporosis in Women (GLOW). J Bone Miner Res 29:260–267
Gregson CL, Dennison EM, Compston JE et al (2014) Disease-specific perception of fracture risk and incident fracture rates: GLOW cohort study. Osteoporos Int 25:85–95
FitzGerald G, Boonen S, Compston JE, Pfeilschifter J, LaCroix AZ, Hosmer DW Jr, Hooven FH, Gehlbach SH (2012) Differing risk profiles for individual fracture sites: evidence from the Global Longitudinal Study of Osteoporosis in Women (GLOW). J Bone Miner Res 27:1907–1915
Hooven FH, Adachi JD, Adami S et al (2009) The Global Longitudinal Study of Osteoporosis in Women (GLOW): rationale and study design. Osteoporos Int 20:1107–1116
Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S (2010) Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res 25:983–993
Funding
We thank the Arthritis Research UK for their support: Arthritis Research UK Grant No. 20380.
Author information
Authors and Affiliations
Contributions
AEL contributed to acquisition, interpretation of data and prepared the first draft of the paper. LDW and DER were responsible for statistical analysis of the data. KW contributed to interpretation of data. Author CC contributed to conception and design of the work. EMD designed the study and contributed to interpretation of data. She is guarantor. All authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Corresponding author
Ethics declarations
Conflict of interest
AE Litwic, LD Westbury, DE Robinson, KA Ward and EM Dennison declare that they have no conflict of interest. C Cooper reports personal fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda and UCB.
Human and Animal Rights and Informed Consent
This research was performed in accordance with the Declaration of Helsinki and was approved by the UK Health Research Authority, reference 07/MRE01/30. All participants gave informed consent.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Litwic, A.E., Westbury, L.D., Robinson, D.E. et al. Bone Phenotype Assessed by HRpQCT and Associations with Fracture Risk in the GLOW Study. Calcif Tissue Int 102, 14–22 (2018). https://doi.org/10.1007/s00223-017-0325-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0325-9