Abstract
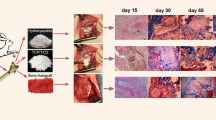
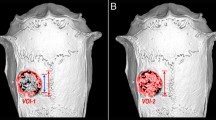
The present study aimed to evaluate and compare the effectiveness of composites of calcium phosphates including β-tri calcium phosphate (β-TCP), dicalcium phosphate anhydrous (DCPA, monetite), mono-calcium phosphate monohydrate (MCPM), and hydroxyapatite (HA) with the chitosan-gelatin-platelet gel (CGP) on the healing of experimentally induced critical size radial bone defects in rats after 8 weeks of injury. Eighty bilateral bone defects were created in the radial bones of 40 adult male Sprague–Dawley rats. The defects were either left empty (untreated or defect group), or treated with autograft, CGP, CGP-DCP, CGP-TCP, CGP/β-TCP/DCPA (CGP-TD), CGP-TD/MCPM (CGP-TDM), and CGP-TDM/HA (CGP-TDMH) scaffolds. The injured forelimbs were evaluated by radiography, gross morphology, three-dimensional computed tomography scanning, histopathology, histomorphometry, scanning electron microscopy, and biomechanical testing. The materials were analyzed using X-ray diffraction to verify the crystalline nature of their structures, and their crystallinity was revealed based on the diffraction peaks achieved from the XRD analysis. The best results were achieved by the CGP-DCP scaffold and the autograft. The CGP-TCP and CGP-TDMH scaffolds were not degraded, while the CGP-DCP, CGP-TDM, CGP-TD, and CGP scaffolds were biodegraded and enhanced bone formation compared with the CGP-TCP and CGP-TDMH groups (P < 0.05). Overall, the CGP-DCP treated defects showed significant improvement in bone formation and union, bone volume, maximum load, and stiffness compared to the CGP group (P < 0.05). It could be concluded that the CGP-DCP scaffold can be considered as a suitable substitute to autograft. In fact, this study demonstrated that DCPA or monetite has high healing potential due to its biocompatibility, biodegradability and biomechanical, osteoconductive and osteoinductive properties of this bioceramic.





Similar content being viewed by others
References
Oryan A, Alidadi S, Moshiri A et al (2014) Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res 9:18
Hokugo A, Ozeki M, Kawakami O et al (2005) Augmented bone regeneration activity of platelet-rich plasma by biodegradable gelatin hydrogel. Tissue Eng 11:1224–1233
Okamoto SI, Ikeda T, Sawamura K et al (2011) Positive effect on bone fusion by the combination of platelet-rich plasma and a gelatin β-tricalcium phosphate sponge: a study using a posterolateral fusion model of lumbar vertebrae in rats. Tissue Eng A 18:157–166
Sheikh Z, Abdallah MN, Hanafi AA et al (2015) Mechanisms of in vivo degradation and resorption of calcium phosphate based biomaterials. Materials 8:7913–7925
Huang Y, Onyeri S, Siewe M et al (2005) In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials 26:7616–7627
Bi L, Cheng W, Fan H et al (2010) Reconstruction of goat tibial defects using an injectable tricalcium phosphate/chitosan in combination with autologous platelet-rich plasma. Biomaterials 31:3201–3211
Moshiri A, Shahrezaee M, Shekarchi B et al (2015) Three-dimensional porous gelapin-simvastatin scaffolds promoted bone defect healing in rabbits. Calcif Tissue Int 96:552–564
Reno C, Lima B, Sousa E et al (2013) Scaffolds of calcium phosphate cement containing chitosan and gelatin. Mater Res 16:1362–1365
Young S, Wong M, Tabata Y et al (2005) Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Controll Release 109:256–274
Lee JY, Nam SH, Im SY et al (2002) Enhanced bone formation by controlled growth factor delivery from chitosan-based biomaterials. J Controll Release 78:187–197
Oryan A, Alidadi S, Moshiri A (2016) Platelet-rich plasma for bone healing and regeneration. Expert Opin Biol Ther 16:213–232
Tsuzuki N, Seo JP, Yamada K et al (2013) The effect of a gelatin β-tricalcium phosphate sponge loaded with mesenchymal stem cells (MSC), bone morphogenic protein-2, and platelet-rich plasma (PRP) on equine articular cartilage defect. Can Vet J 54:73
Yamada N, Kim WC, Yoshida T et al (2013) Effect of gelatin β-tricalcium phosphate sponge with platelet-rich plasma on bone regeneration in massive ulna defects. J Med Biol Eng 33:545–551
Yin Y, Ye F, Cui J et al (2003) Preparation and characterization of macroporous chitosan-gelatin/β-tricalcium phosphate composite scaffolds for bone tissue engineering. J Biomed Mater Res A 67:844–855
Cheng L, Ye F, Yang R et al (2010) Osteoinduction of hydroxyapatite/β-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomater 6:1569–1574
Bose S, Tarafder S (2012) Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater 8:1401–1421
Dorozhkin SV (2009) Calcium orthophosphates in nature, biology and medicine. Materials 2:399–498
Gbureck U, Holzel T, Klammert U et al (2007) Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. Adv Funct Mater 17:3940–3945
Montazerolghaem M, Ott MK, Engqvist H et al (2015) Resorption of monetite calcium phosphate cement by mouse bone marrow derived osteoclasts. Mater Sci Eng C 52:212–218
Theiss F, Apelt D, Brand B (2005) Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials 26:4383–4394
Ohura K, Bohner M, Hardouin P (1999) Resorption of, and bone formation from, new beta-tricalcium phosphate-monocalcium phosphate cements: an in vivo study. J Biomed Mater Res 30:193–200
Danilchenko SN, Kalinkevich OV, Pogorelov MW (2011) Characterization and in vivo evaluation of chitosan-hydroxyapatite bone scaffolds made by one step coprecipitation method. J Biomed Mater Res A 96:639–647
Zhou Y, Xu L, Zhang X et al (2012) Radiation synthesis of gelatin/CM-chitosan/β-tricalcium phosphate composite scaffold for bone tissue engineering. Mater Sci Eng C 32:994–1000
Moshiri A, Oryan A, Meimandi-Parizi A (2015) Synthesis, development, characterization and effectiveness of bovine pure platelet gel-collagen-polydioxanone bioactive graft on tendon healing. J Cell Mol Med 19:1308–1332
Oryan A, Parizi AM, Shafiei-Sarvestani Z et al (2012) Effects of combined hydroxyapatite and human platelet rich plasma on bone healing in rabbit model: radiological, macroscopical, hidtopathological and biomechanical evaluation. Cell Tissue Bank 13:639–651
Parizi AM, Oryan A, Shafiei-Sarvestani Z et al (2012) Human platelet rich plasma plus Persian Gulf coral effects on experimental bone healing in rabbit model: radiological, histological, macroscopical and biomechanical evaluation. J Mater Sci Mater Med 23:473–483
Oryan A, Alidadi S, Bigham-Sadegh A et al (2016) Comparative study on the role of gelatin, chitosan and their combination as tissue engineered scaffolds on healing and regeneration of critical sized bone defects: an in vivo study. J Mater Sci Mater Med 27:155
Parizi AM, Oryan A, Shafiei-Sarvestani Z et al (2013) Effectiveness of synthetic hydroxyapatite versus Persian Gulf coral in an animal model of long bone defect reconstruction. J Orthop Trauma 14:259–268
Shafiei-Sarvestani Z, Oryan A, Bigham AS et al (2012) The effect of hydroxyapatite-hPRP, and coral-hPRP on bone healing in rabbits: radiological, biomechanical, macroscopic and histopathologic evaluation. Int J Surg 10:96–101
Sunil P, Goel S, Rastogi A et al (2008) Incorporation and biodegradation of hydroxyapatite-tricalcium phosphate implanted in large metaphyseal defects–an animal study. Indian J Exp Biol 46:836
Tamimi F, Torres J, Kathan C et al (2008) Bone regeneration in rabbit calvaria with novel monetite granules. J Biomed Mater Res A 87:980–985
Sheikh Z, Zhang YL, Grover L et al (2015) In vitro degradation and in vivo resorption of dicalcium phosphate cement based grafts. Acta Biomater 26:338–346
Sheikh Z, Zhang YL, Tamimi F et al (2017) Effect of processing conditions of dicalcium phosphate cements on graft resorption and bone formation. Acta Biomater 53:526–535
Monchau F, Lefevre A, Descamps M et al (2002) In vitro studies of human and rat osteoclast activity on hydroxyapatite, β-tricalcium phosphate, calcium carbonate. Biomol Eng 19:143–152
Alge DL, Goebel WS, Chu TMG (2013) Effects of DCPD cement chemistry on degradation properties and cytocompatibility: comparison of MCPM/β-TCP and MCPM/HA formulations. Biomed Mater 8:025010
Huan Z, Chang J (2009) Novel bioactive composite bone cements based on the β-tricalcium phosphate-monocalcium phosphate monohydrate composite cement system. Acta Biomater 5:1253–1264
Tamimi F, Le Nihouannen D, Eimar H et al (2012) The effect of autoclaving on the physical and biological properties of dicalcium phosphate dihydrate bioceramics: brushite vs. monetite. Acta Biomater 8:3161–3169
Sheikh Z, Zhang YL, Grover L et al (2015) In vitro degradation and in vivo resorption of dicalcium phosphate cement based grafts. Acta Biomater 26:338–346
Tamimi F, Sheikh Z, Barralet J (2012) Dicalcium phosphate cements: brushite and monetite. Acta Biomater 8:474–487
Tamimi F, Torres J, Gbureck U et al (2009) Craniofacial vertical bone augmentation: a comparison between 3D printed monolithic monetite blocks and autologous onlay grafts in the rabbit. Biomaterials 30:6318–6326
Ohura K, Hamanishi C, Tanaka S et al (1999) Healing of segmental bone defects in rats induced by a β-TCP-MCPM cement combined with rhBMP-2. J Biomed Mater Res 44:168–175
Acknowledgements
The authors would like to thank the authorities of Shiraz University for their kind cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ahmad Oryan, Soodeh Alidadi, and Amin Bigham-Sadegh declare that they have no conflict of interest and financial disclosure associated with this research or its contents.
Human and Animal Rights and Informed Consent
All the animals received humane care based on the Guide for Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1985). The local Ethics Committee of “Regulations for using animals in scientific procedures” in our university approved this experiment.
Rights and permissions
About this article
Cite this article
Oryan, A., Alidadi, S. & Bigham-Sadegh, A. Dicalcium Phosphate Anhydrous: An Appropriate Bioceramic in Regeneration of Critical-Sized Radial Bone Defects in Rats. Calcif Tissue Int 101, 530–544 (2017). https://doi.org/10.1007/s00223-017-0309-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0309-9