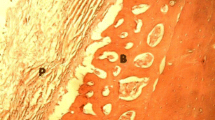
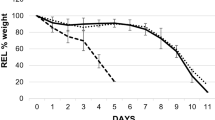
Abstract
Coral is an osteoconductive material used as a bone graft extender and human platelet rich plasma has been used as a source of osteoinductive factor. A combination of human platelet rich plasma and coral is expected to create a composite with both osteoconductive and osteoinductive properties. This study examined the effect of a combination of human platelet rich plasma and coral on osteogenesis in vivo using rabbit model of bone healing. A critical size defect of 10 mm elongation was created in the radial diaphysis of 36 rabbit and either supplied with coral-human PRP, or coral alone or left empty (control group). The platelets in the PRP were about 10.1 fold compared to normal blood. Radiographs of each forelimb was taken postoperatively on 1st day and then at the 2nd, 4th, 6th and 8th weeks post injury to evaluate bone formation, union and remodeling of the defect. The operated radiuses were removed on 56th postoperative day and were grossly and histopathologically evaluated. In addition, biomechanical test was conducted on the operated and normal forearms of the rabbits. This study demonstrated that coral-human PRP (hPRP), could promote bone regeneration in critical size defects with a high regenerative capacity. The results of the present study demonstrated that coral-hPRP could be an attractive alternative for reconstruction of the major diaphyseal defects of the long bones in animal models.










Similar content being viewed by others
References
Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;329:300–9.
Damien C, Parsons R. Bone graft and bone graft substitutes: review of current technology and applications. J Appl Biomater. 1991;2:187–208.
Van heest A, Swiontkowski M. Bone-graft substitutes. Lancet. 1999;353:28–9.
Friedlaender GE. Bone grafts: the basic science rationale for clinical applications. J Bone Joint Surg Am. 1987;69:786–90.
Inoue K, Ohgushi H, Yoshikawa T, Okumura M, Sempuku T, Tamai S, et al. The effect of aging on bone formation in porous hydroxyapatite: biochemical and histologic analysis. J Bone Miner Res. 1997;12:989–94.
Albrek T, Johansson C. Osteoinduction, osteoconduction and osteointegration. Eur Spine J. 2001;10:S96–101.
Alexander JW. Leonard’s orthopedic surgery of the dog and cat. 3rd ed. Florida: WB Sounders Company; 1985.
Alexander JW. Bone grafting. Vet Clin of North Am Small Anim Pract. 1987;17(4):811–9.
Brinker WO, Piermattei DL, Flo GL. Bone grafting. Small animal orthopedics and fracture repair. 3rd ed. Florida: WB Saunders Company; 1997. p. 147–53.
Fitch R, Kerwin S, Newman-Gage H, Sinibaldi KR. Bone autografts and allografts in dogs. Comp Vet Cont Ed. 1997;19(5):558–75.
Fox SM. Cancellous bone grafting in the dog: an overview. J Am Anim Hosp Assoc. 1984;20:840–8.
McLaughlin RM, Roush JK. Autogenous cancellous and cortico-cancellous bone grafting. Vet Med. 1998;93(12):1071–4.
Lohmann CH, Andreacchio D, Koster G, Carnes DL, Dean BD, Schwartz Z. Tissue response and osteoinduction of human bone grafts in vivo. Arch Orthop Trauma Surg. 2001;121:583–90.
Pokorny JJ, Davids H, Moneim M. Vascularized bone graft for scaphoid nonunion. Tech Hand Up Extrem Surg. 2003;7:32–6.
Bauer TW, Muschler GF. Bone graft materials: an overview of the basic science. Clin Orthop Rel Res. 2000;371:10–27.
Keating JF, McQueen MM. Substitutes for autologous bone graft in orthopaedic trauma. J Bone Joint Surg Am. 2001;83-B:3–8.
Kim DH, Jenis L, Berta SC, Vaccaro AR. Bone graft alternatives in spinal fusion surgery. Cur Opin in Orthop. 2003;14:127–37.
Emami MJ, Oryan A, Saeidinasab H, Meimandi Parizi A. The effect of bone marrow graft on bone healing: a radiological and biomechanical study. Iran J Med Sci. 2002;27:63–6.
Meimandi Parizi A, Jelodar G, Moslemi H, KT A, Emami MJ. Influence of hydroxyapatite on fracture healing in diabetic rats: biomechanical and radiographic studies. Veterinarski Arhiv. 2010;80:113–20.
Meimandi Parizi A, Zeidabadi nejad GR. Biomechanical and radiographical evaluation of the effects of constant direct current on the fracture healing of the radius in the rabbits. J Fac Vet Med Univ Tehran. 1997;52:1–10.
Hollinger JO, Brekke J, Gruskin E, Lee D. Role of bone substitutes. Clin Orthop Relat Res. 1996;324:55–65.
Bostrom MP, Saleh KJ, Einhorn TA. Osteoinductive growth factors in preclinical fracture and long bone defects models. Orthop Clin North Am. 1999;30:647–58.
Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97–102.
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46.
McClain SA, Simon M, Jones E, Nandi A, Gailit JO, Tonnesen MG. Mesenchymal cell activation is the rate-limiting step of granulation tissue induction. Am J Pathol. 1996;149:1257–70.
Mustoe TA, Pierce GF, Morishima C, Deuel TF. Growth factorinduced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest. 1991;87:694–703.
Saba AA, Freedman BM, Gaffield JW, Mackay DR, Ehrlich HP. Topical platelet-derived growth factor enhances wound closure in the absence of wound contraction: an experimental and clinical study. Ann Plast Surg. 2002;49:62–6.
Aghaloo TL, Moy PK, Freymiller EG. Investigation of platelet-rich plasma in rabbit cranial defects: a pilot study. J Oral Maxillofac Surg. 2002;60:1176–81.
Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–35.
Kassolis JD, Rosen PS, Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. J Periodontol. 2000;71:1654–61.
Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15:203–8.
Robiony M, Polini F, Costa F, Politi M. Osteogenesis distraction and platelet-rich plasma for bone restoration of the severely atrophic mandible: preliminary results. J Oral Maxillofac Surg. 2002;60:630–5.
Rodriguez A, Anastassov GE, Lee H, Buchbinder D, Wettan H. Maxillary sinus augmentation with deproteinated bovine bone and platelet rich plasma with simultaneous insertion of endosseous implants. J Oral Maxillofac Surg. 2003;61:157–63.
Schlegel KA, Donath K, Rupprecht S, Falk S, Zimmermann R, Felszeghy E. De novo bone formation using bovine collagen and platelet-rich plasma. Biomaterials. 2004;25:5387–93.
Froum SJ, Wallace SS, Tarnow DP, Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodont Restor Dent. 2002;22:45–53.
Kim SG, Kim WK, Park JC, Kim HJ. A comparative study of osseointegration of Avana implants in a demineralized freeze-dried bone alone or with platelet-rich plasma. J Oral Maxillofac Surg. 2002;60:1018–25.
Bouchon C, Lebrun T, Rouvillain JL, Roudier M. The Caribbean Scleractinian corals used for surgical implants. Bull Inst Océanogr. 1995;14(3):111–22.
Kavousi J, Seyfabadi J, Rezai H, Fenner D. Coral reefs and communities of Qeshm Island, the Persian Gulf. Zool Stud. 2011;50(3):276–83.
Ghavam Mostafavi P, Fatemi SMR, Shahhosseiny MH, Hoegh-Guldberg O, WLW K. Predominance of clade D Symbiodinium in shallow-water reef-building corals of Kish and Larak Islands (Persian Gulf, Iran). Mar Biol. 2007;153:25–34.
Guillemin G, Patat JL, Fournie J, Chetail M. The use of coral as a bone graft substitute. J Biomed Mater Res. 1987;21(5):557–67.
Guillemin G, Meunier A, Dallant P, Christel P, Pouliquen J. Comparison of coral resorption and bone apposition with two natural corals of different porosities. J Biomed Mater Res. 1989;23(7):765–79.
Irigaray JL, Oudadesse H, El FH. Effet de la température sur la structure cristalline d’un Biocorail. J Therm Anal. 1993;39:3–14.
Lane JM, Sandhu HS. Current approach to experimental bone grafting. Orthop Clin North Am. 1987;18:213–25.
Emery SE, Brazinski MS, Koka A, Bensusan JS, Stevenson S. The biological and biomechanical effects of irradiation on anterior spinal bone grafts in a canine model. J Bone Jt Surg. 1994;76(4):540.
An YH, Friedman RJ. Animal models in orthopedic research. 1st ed. Boca Raton: CRC Press Inc.; 1999.
Bolander ME, Galian G. The use of demineralize bone matrix in the repair of segmental defect. J Bone Jt Surg. 1983;68A:1264–74.
Thorwarth M, Rupprecht S, Falk S, Felszeghy E, Wiltfang J, Schlegel KA. Expression of bone matrix proteins during de novo bone formation using a bovine collagen and platelet-rich plasma (prp)-an immunohistochemical analysis. Biomaterials. 2005;26:2575–84.
Shanaman R, Filstein MR, Danesh-Meyer MJ. Localized ridge augmentation using GBR and platelet-rich plasma: case reports. Int J Oral Maxillofac Implants Periodont Restor Dent. 2001;21:345–55.
Raghoebar GM, Schortinghuis J, Liem RS, Ruben JL, van der Wal JE, Vissink A. Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin Oral Implants Res. 2005;16:349–56.
Grageda E. Platelet-rich plasma and bone graft materials:a review and a standardized research protocol. Implant Dent. 2004;13(4):301–9.
Papa F, Cortese A, Sagliocco R, Farella M, Banzi C, Maltarello MC, et al. Outcome of 47 consecutive sinus lift operations using aragonitic calcium carbonate associated with autologous platelet-rich plasma: clinical, histologic, and histomorphometrical evaluations. J Craniofac Surg. 2009;20(6):2067.
Zhang Y, Wang Y, Shi B, Cheng X. A platelet-derived growth factor releasing chitosan/coral composite scaffold for periodontal tissue engineering. Biomaterials. 2007;28(8):1515–22.
Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–96.
Pouliquen JC, Noat M, Verneret C, Guillemin G, Patat J. Coral as a substitute for bone graft in posterior spine fusion in childhood. Fr J Orthop Surg. 1989;3:272–80.
Zajour W, Dehoux E, Deprey F, Segal P. Use of coral as a bone graft substitute for anterior fusion of lower spine. Orthop Prod News. 1992:38–9.
Roux FX, Brasnu D, Loty B, George B, Guillemin G. Madreporic coral: a new bone graft substitute for cranial surgery. J Neurosurg. 1988;69(4):510–3.
Yukna RA. Clinical evaluation of coralline calcium carbonate as a bone replacement graft material in human periodontal osseous defects. J Periodontol. 1994;65:177–85.
Ohgushi H, Goldberg VM, Caplan AI. Repair of bone defects with marrow cells and porous ceramic: experiments in rats. Acta Orthop Scand. 1989;60:334–9.
Ohgushi H, Okumura M, Yoshikawa T, Inboue K, Senpuku N, Tamai S, et al. Bone formation processin porous calcium carbonate and hydroxyapatite. J Biomed Mater Res. 1992;26(7):885–95.
Vuola J, Goransson H, Bohling T, Asko-Seljavaara S. Bone marrow induced osteogenesis in hydroxyapatite and calcium carbonate implants. Biomaterials. 1996;17(18):1761–6.
Petite H, Kacem K, Triffitt JT. Adhesion, growth and differentiation of human bone marrow stromal cells on non-porous calcium carbonate and plastic substrata: effects of dexamethasone and 1, 25 dihydroxyvitamin D3. J Mater Sci Mater in Med. 1996;7:665–71.
Assoian RK, Grotendorst GR, Miller DM, Sporn MB. Cellular transformation by coordinated action of three peptide growth factors from human platelets. Nature. 1984;309:804–6.
Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87:305–12.
Baylink DJ, Finkelman RD, Mohan S. Growth factors to stimulate bone formation. J Bone Miner Res. 1993;8:565–72.
Spencer EM, Liu CC, Si EC, Howard GA. In vivo actions of insulinlike growth factor-I (IGF-I) on bone formation and resorption in rats. Bone. 1991;12:21–6.
Street J, Bao M, deGuzman L, Bunting S, Peale JF, Ferrara N. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–61.
Cook SD. Preclinical and clinical evaluation of osteogenic protein-1 (BMP-7) in bony sites. Orthopedics. 1999;22:669–71.
Plachokova AS, van den Dolder J, van den Beucken JJJP, Jansen JA. Bone regenerative properties of rat, goat and human platelet-rich plasma. Int J Oral Maxillofac Implants. 2009;38(8):861–9.
Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15(2):203–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parizi, A.M., Oryan, A., Shafiei-Sarvestani, Z. et al. Human platelet rich plasma plus Persian Gulf coral effects on experimental bone healing in rabbit model: radiological, histological, macroscopical and biomechanical evaluation. J Mater Sci: Mater Med 23, 473–483 (2012). https://doi.org/10.1007/s10856-011-4478-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4478-1