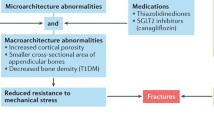
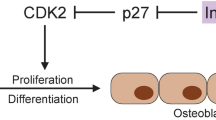
Abstract
It has been well established that bone fragility is one of the chronic complications of diabetes mellitus, and both type 1 and type 2 diabetes are risk factors for fragility fractures. Diabetes may negatively affect bone health by unbalancing several pathways: bone formation, bone resorption, collagen formation, inflammatory cytokine, muscular and incretin system, bone marrow adiposity and calcium metabolism. The purpose of this narrative review is to explore the current understanding of pathophysiological pathways underlying bone fragility in diabetics. In particular, the review will focus on the peculiar cellular and molecular system impairment that may lead to increased risk of fracture in type 1 and type 2 diabetes.

Similar content being viewed by others
References
Napoli N, Chandran M, Pierroz DD et al (2016) Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. doi:10.1038/nrendo.2016.153
Ferron M, Wei J, Yoshizawa T et al (2010) Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142:296–308. doi:10.1016/j.cell.2010.06.003
Napoli N, Schafer AL, Lui L-Y et al (2016) Serum 25-hydroxyvitamin D level and incident type 2 diabetes in older men, the osteoporotic fractures in men (MrOS) study. Bone 90:181–184. doi:10.1016/j.bone.2016.07.001
Napoli N, Strotmeyer ES, Ensrud KE et al (2014) Fracture risk in diabetic elderly men: the MrOS study. Diabetologia 57:2057–2065. doi:10.1007/s00125-014-3289-6
Pun KK, Lau P, Ho PW (1989) The characterization, regulation, and function of insulin receptors on osteoblast-like clonal osteosarcoma cell line. J Bone Miner Res 4:853–862. doi:10.1002/jbmr.5650040610
Cornish J, Callon KE, Reid IR (1996) Insulin increases histomorphometric indices of bone formation In vivo. Calcif Tissue Int 59:492–495
Fulzele K, Riddle RC, DiGirolamo DJ et al (2010) Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142:309–319. doi:10.1016/j.cell.2010.06.002
Lee YH, White MF (2004) Insulin receptor substrate proteins and diabetes. Arch Pharm Res 27:361–370
Ogata N, Chikazu D, Kubota N et al (2000) Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J Clin Invest 105:935–943. doi:10.1172/JCI9017
Bu Y-H, He Y-L, Zhou H-D et al (2010) Insulin receptor substrate 1 regulates the cellular differentiation and the matrix metallopeptidase expression of preosteoblastic cells. J Endocrinol 206:271–277. doi:10.1677/JOE-10-0064
Shimoaka T, Kamekura S, Chikuda H et al (2004) Impairment of bone healing by insulin receptor substrate-1 deficiency. J Biol Chem 279:15314–15322. doi:10.1074/jbc.M312525200
Hie M, Tsukamoto I (2010) Increased expression of the receptor for activation of NF-kappaB and decreased runt-related transcription factor 2 expression in bone of rats with streptozotocin-induced diabetes. Int J Mol Med 26:611–618
Campos Pastor MM, López-Ibarra PJ, Escobar-Jiménez F et al (2000) Intensive insulin therapy and bone mineral density in type 1 diabetes mellitus: a prospective study. Osteoporos Int 11:455–459
Fulzele K, DiGirolamo DJ, Liu Z et al (2007) Disruption of the insulin-like growth factor type 1 receptor in osteoblasts enhances insulin signaling and action. J Biol Chem 282:25649–25658. doi:10.1074/jbc.M700651200
Moyer-Mileur LJ, Slater H, Jordan KC, Murray MA (2008) IGF-1 and IGF-binding proteins and bone mass, geometry, and strength: relation to metabolic control in adolescent girls with type 1 diabetes. J Bone Miner Res 23:1884–1891. doi:10.1359/jbmr.080713
Ardawi M-SM, Akhbar DH, Alshaikh A et al (2013) Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone 56:355–362. doi:10.1016/j.bone.2013.06.029
Barrett-Connor E, Kritz-Silverstein D (1996) Does hyperinsulinemia preserve bone? Diabetes Care 19:1388–1392
Stolk RP, Van Daele PL, Pols HA et al (1996) Hyperinsulinemia and bone mineral density in an elderly population: the Rotterdam study. Bone 18:545–549
Christensen JD, Lungu AO, Cochran E et al (2014) Bone mineral content in patients with congenital generalized lipodystrophy is unaffected by metreleptin replacement therapy. J Clin Endocrinol Metab 99:E1493–E1500. doi:10.1210/jc.2014-1353
Wei J, Ferron M, Clarke CJ et al (2014) Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest 124:1–13. doi:10.1172/JCI72323
de Liefde II, van der Klift M, de Laet CEDH et al (2005) Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam study. Osteoporos Int 16:1713–1720. doi:10.1007/s00198-005-1909-1
Rinker TE, Hammoudi TM, Kemp ML et al (2014) Interactions between mesenchymal stem cells, adipocytes, and osteoblasts in a 3D tri-culture model of hyperglycemic conditions in the bone marrow microenvironment. Integr Biol (Camb) 6:324–337. doi:10.1039/c3ib40194d
Zhao Y-F, Zeng D-L, Xia L-G et al (2013) Osteogenic potential of bone marrow stromal cells derived from streptozotocin-induced diabetic rats. Int J Mol Med 31:614–620. doi:10.3892/ijmm.2013.1227
Hie M, Iitsuka N, Otsuka T, Tsukamoto I (2011) Insulin-dependent diabetes mellitus decreases osteoblastogenesis associated with the inhibition of Wnt signaling through increased expression of Sost and Dkk1 and inhibition of Akt activation. Int J Mol Med 28:455–462. doi:10.3892/ijmm.2011.697
Fu C, Zhang X, Ye F, Yang J (2015) High insulin levels in KK-Ay diabetic mice cause increased cortical bone mass and impaired trabecular micro-structure. Int J Mol Sci 16:8213–8226. doi:10.3390/ijms16048213
Hie M, Shimono M, Fujii K, Tsukamoto I (2007) Increased cathepsin K and tartrate-resistant acid phosphatase expression in bone of streptozotocin-induced diabetic rats. Bone 41:1045–1050. doi:10.1016/j.bone.2007.08.030
Fujii H, Hamada Y, Fukagawa M (2008) Bone formation in spontaneously diabetic Torii-newly established model of non-obese type 2 diabetes rats. Bone 42:372–379. doi:10.1016/j.bone.2007.10.007
Won HY, Lee J-A, Park ZS et al (2011) Prominent bone loss mediated by RANKL and IL-17 produced by CD4+ T cells in TallyHo/JngJ mice. PLoS One 6:e18168. doi:10.1371/journal.pone.0018168
Wang A, Midura RJ, Vasanji A et al (2014) Hyperglycemia diverts dividing osteoblastic precursor cells to an adipogenic pathway and induces synthesis of a hyaluronan matrix that is adhesive for monocytes. J Biol Chem 289:11410–11420. doi:10.1074/jbc.M113.541458
García-Hernández A, Arzate H, Gil-Chavarría I et al (2012) High glucose concentrations alter the biomineralization process in human osteoblastic cells. Bone 50:276–288. doi:10.1016/j.bone.2011.10.032
Ehnert S, Freude T, Ihle C et al (2015) Factors circulating in the blood of type 2 diabetes mellitus patients affect osteoblast maturation - description of a novel in vitro model. Exp Cell Res 332:247–258. doi:10.1016/j.yexcr.2014.12.011
Okazaki K, Yamaguchi T, Tanaka K-I et al (2012) Advanced glycation end products (AGEs), but not high glucose, inhibit the osteoblastic differentiation of mouse stromal ST2 cells through the suppression of osterix expression, and inhibit cell growth and increasing cell apoptosis. Calcif Tissue Int 91:286–296. doi:10.1007/s00223-012-9641-2
Kim JH, Kim Y-Y, Kim S-J (2009) High glucose inhibits gene expression of tyrosyl-tRNA synthetase in osteoblast cells. Methods Find Exp Clin Pharmacol 31:639–644. doi:10.1358/mf.2009.31.10.1441114
Manavalan JS, Cremers S, Dempster DW et al (2012) Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab 97:3240–3250. doi:10.1210/jc.2012-1546
Tanaka K, Yamaguchi T, Kanazawa I, Sugimoto T (2015) Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun 461:193–199. doi:10.1016/j.bbrc.2015.02.091
Kang J, Boonanantanasarn K, Baek K et al (2015) Hyperglycemia increases the expression levels of sclerostin in a reactive oxygen species- and tumor necrosis factor-alpha-dependent manner. J Periodontal Implant Sci 45:101–110. doi:10.5051/jpis.2015.45.3.101
Neumann T, Hofbauer LC, Rauner M et al (2014) Clinical and endocrine correlates of circulating sclerostin levels in patients with type 1 diabetes mellitus. Clin Endocrinol (Oxf) 80:649–655. doi:10.1111/cen.12364
Xu J, Yue F, Wang J et al (2015) High glucose inhibits receptor activator of nuclear factor- κB ligand-induced osteoclast differentiation via downregulation of v- ATPase V0 subunit d2 and dendritic cell-specific transmembrane protein. Mol Med Rep 11:865–870. doi:10.3892/mmr.2014.2807
Kasahara T, Imai S, Kojima H et al (2010) Malfunction of bone marrow-derived osteoclasts and the delay of bone fracture healing in diabetic mice. Bone 47:617–625. doi:10.1016/j.bone.2010.06.014
Wittrant Y, Gorin Y, Woodruff K et al (2008) High d(+)glucose concentration inhibits RANKL-induced osteoclastogenesis. Bone 42:1122–1130. doi:10.1016/j.bone.2008.02.006
Xu F, Ye Y, Dong Y et al (2013) Inhibitory effects of high glucose/insulin environment on osteoclast formation and resorption in vitro. J Huazhong Univ Sci Technol Med Sci 33:244–249. doi:10.1007/s11596-013-1105-z
Hamann C, Goettsch C, Mettelsiefen J et al (2011) Delayed bone regeneration and low bone mass in a rat model of insulin-resistant type 2 diabetes mellitus is due to impaired osteoblast function. Am J Physiol Endocrinol Metab 301:E1220–E1228. doi:10.1152/ajpendo.00378.2011
Saito M, Soshi S, Tanaka T, Fujii K (2004) Intensity-related differences in collagen post-translational modification in MC3T3-E1 osteoblasts after exposure to low- and high-intensity pulsed ultrasound. Bone 35:644–655. doi:10.1016/j.bone.2004.04.024
Saito M, Marumo K (2013) Bone quality in diabetes. Front Endocrinol (Lausanne) 4:72. doi:10.3389/fendo.2013.00072
Turecek C, Fratzl-Zelman N, Rumpler M et al (2008) Collagen cross-linking influences osteoblastic differentiation. Calcif Tissue Int 82:392–400. doi:10.1007/s00223-008-9136-3
Raposo B, Rodríguez C, Martínez-González J, Badimon L (2004) High levels of homocysteine inhibit lysyl oxidase (LOX) and downregulate LOX expression in vascular endothelial cells. Atherosclerosis 177:1–8. doi:10.1016/j.atherosclerosis.2004.06.015
Leslie WD, Rubin MR, Schwartz AV, Kanis JA (2012) Type 2 diabetes and bone. J Bone Miner Res 27:2231–2237. doi:10.1002/jbmr.1759
Cui S, Xiong F, Hong Y et al (2011) APPswe/Aβ regulation of osteoclast activation and RAGE expression in an age-dependent manner. J Bone Miner Res 26:1084–1098. doi:10.1002/jbmr.299
Saito M, Fujii K, Mori Y, Marumo K (2006) Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int 17:1514–1523. doi:10.1007/s00198-006-0155-5
Silva MJ, Brodt MD, Lynch MA et al (2009) Type 1 diabetes in young rats leads to progressive trabecular bone loss, cessation of cortical bone growth, and diminished whole bone strength and fatigue life. J Bone Miner Res 24:1618–1627. doi:10.1359/jbmr.090316
Okazaki R, Totsuka Y, Hamano K et al (1997) Metabolic improvement of poorly controlled noninsulin-dependent diabetes mellitus decreases bone turnover. J Clin Endocrinol Metab 82:2915–2920. doi:10.1210/jcem.82.9.4258
Yamamoto M, Yamaguchi T, Yamauchi M et al (2008) Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 93:1013–1019. doi:10.1210/jc.2007-1270
Neumann T, Lodes S, Kästner B et al (2014) High serum pentosidine but not esRAGE is associated with prevalent fractures in type 1 diabetes independent of bone mineral density and glycaemic control. Osteoporos Int 25:1527–1533. doi:10.1007/s00198-014-2631-7
Dorrian CA, Cathcart S, Clausen J, et al. (1998) Factors in human serum interfere with the measurement of advanced glycation endproducts. Cell Mol Biol (Noisy-le-grand) 44:1069–1079.
Baggio LL, Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157. doi:10.1053/j.gastro.2007.03.054
Zhong Q, Itokawa T, Sridhar S et al (2007) Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am J Physiol Endocrinol Metab 292:E543–E548. doi:10.1152/ajpendo.00364.2006
Nissen A, Christensen M, Knop FK et al (2014) Glucose-dependent insulinotropic polypeptide inhibits bone resorption in humans. J Clin Endocrinol Metab 99:E2325–E2329. doi:10.1210/jc.2014-2547
Faienza MF, Luce V, Ventura A et al (2015) Skeleton and glucose metabolism: a bone-pancreas loop. Int J Endocrinol 2015:758148. doi:10.1155/2015/758148
Tsukiyama K, Yamada Y, Yamada C et al (2006) Gastric Inhibitory Polypeptide as an Endogenous Factor Promoting New Bone Formation after Food Ingestion. Mol Endocrinol 20:1644–1651. doi:10.1210/me.2005-0187
Mieczkowska A, Irwin N, Flatt PR et al (2013) Glucose-dependent insulinotropic polypeptide (GIP) receptor deletion leads to reduced bone strength and quality. Bone 56:337–342. doi:10.1016/j.bone.2013.07.003
Nuche-Berenguer B, Portal-Núñez S, Moreno P et al (2010) Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J Cell Physiol 225:585–592. doi:10.1002/jcp.22243
Jeon YK, Bae MJ, Kim JI et al. (2014) Expression of Glucagon-Like Peptide 1 Receptor during Osteogenic Differentiation of Adipose-Derived Stem Cells. Endocrinol Metab 29:567–573. doi:10.3803/EnM.2014.29.4.567
Gilbert MP, Pratley RE (2015) The impact of diabetes and diabetes medications on bone health. Endocr Rev 36:194–213. doi:10.1210/er.2012-1042
Nuche-Berenguer B, Moreno P, Esbrit P et al (2009) Effect of GLP-1 treatment on bone turnover in normal, type 2 diabetic, and insulin-resistant states. Calcif Tissue Int 84:453–461. doi:10.1007/s00223-009-9220-3
Mansur SA, Mieczkowska A, Bouvard B et al (2015) Stable Incretin Mimetics Counter Rapid Deterioration of Bone Quality in Type 1 Diabetes Mellitus. J Cell Physiol 230:3009–3018. doi:10.1002/jcp.25033
Iepsen EW, Lundgren JR, Hartmann B et al (2015) GLP-1 receptor agonist treatment increases bone formation and prevents bone loss in weight-reduced obese women. J Clin Endocrinol Metab 100:2909–2917. doi:10.1210/jc.2015-1176
Conte C, Cecere A, Guglielmi G, Napoli N (2015) Letter to the editor: “GLP-1 receptor agonist treatment increases bone formation and prevents bone loss in weight-reduced obese women” by Iepsen E.W., et al. J Clin Endocrinol Metab 100:L92–L93. doi:10.1210/jc.2015-2970
Zhao Y, Kachroo S, Kawabata H et al (2016) Association between hypoglycemia and fall-related fractures and health care utilization in older veterans with type 2 diabetes. Endocr Pract 22:196–204. doi:10.4158/EP15640.OR
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int 18:427–444. doi:10.1007/s00198-006-0253-4
Khan TS, Fraser L-A (2015) Type 1 diabetes and osteoporosis: from molecular pathways to bone phenotype. J Osteoporos 2015:174186. doi:10.1155/2015/174186
Formiga F, Chivite D, Ruiz D et al (2015) Clinical evidence of diabetes mellitus end-organ damage as risk factor for falls complicated by hip fracture: a multi-center study of 1225 patients. Diabetes Res Clin Pract 109:233–237. doi:10.1016/j.diabres.2015.05.050
Shanbhogue VV, Hansen S, Frost M et al (2015) Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol 174:115–124. doi:10.1530/EJE-15-0860
Napoli N, Strollo R, Pitocco D et al (2013) Effect of calcitriol on bone turnover and osteocalcin in recent-onset type 1 diabetes. PLoS ONE 8:e56488. doi:10.1371/journal.pone.0056488
Cutrim DMSL, Pereira FA, de Paula FJA, Foss MC (2007) Lack of relationship between glycemic control and bone mineral density in type 2 diabetes mellitus. Brazilian J Med Biol Res 40:221–7
Yamamoto M, Yamaguchi T, Nawata K et al (2012) Decreased PTH levels accompanied by low bone formation are associated with vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 97:1277–1284. doi:10.1210/jc.2011-2537
Thalassinos NC, Hadjiyanni P, Tzanela M et al (1993) Calcium metabolism in diabetes mellitus: effect of improved blood glucose control. Diabet Med 10:341–344
Jules J, Feng X (2014) In vitro investigation of the roles of the proinflammatory cytokines tumor necrosis factor-α and interleukin-1 in murine osteoclastogenesis. Methods Mol Biol 1155:109–123. doi:10.1007/978-1-4939-0669-7_10
Diarra D, Stolina M, Polzer K et al (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13:156–163. doi:10.1038/nm1538
Roggia C, Gao Y, Cenci S et al (2001) Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A 98:13960–13965. doi:10.1073/pnas.251534698
Kristiansen OP, Mandrup-Poulsen T (2005) Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes 54(Suppl 2):S114–S124
Park JH, Park KH, Cho S et al (2013) Concomitant increase in muscle strength and bone mineral density with decreasing IL-6 levels after combination therapy with alendronate and calcitriol in postmenopausal women. Menopause 20:747–753. doi:10.1097/GME.0b013e31827cabca
Aguirre L, Napoli N, Waters D et al (2014) Increasing adiposity is associated with higher adipokine levels and lower bone mineral density in obese older adults. J Clin Endocrinol Metab 99:3290–3297. doi:10.1210/jc.2013-3200
Weyer C, Funahashi T, Tanaka S et al (2001) Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86:1930–1935. doi:10.1210/jcem.86.5.7463
Williams GA, Wang Y, Callon KE et al (2009) In vitro and in vivo effects of adiponectin on bone. Endocrinology 150:3603–3610. doi:10.1210/en.2008-1639
Napoli N, Pedone C, Pozzilli P et al (2010) Adiponectin and bone mass density: the InCHIANTI study. Bone 47:1001–1005. doi:10.1016/j.bone.2010.08.010
Napoli N, Strollo R, Paladini A et al (2014) The alliance of mesenchymal stem cells, bone, and diabetes. Int J Endocrinol 2014:690783. doi:10.1155/2014/690783
Nouh MR, Eid AF (2015) Magnetic resonance imaging of the spinal marrow: basic understanding of the normal marrow pattern and its variant. World J Radiol 7:448–458. doi:10.4329/wjr.v7.i12.448
Patsch JM, Li X, Baum T et al (2013) Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res 28:1721–1728. doi:10.1002/jbmr.1950
Piccinin MA, Khan ZA (2014) Pathophysiological role of enhanced bone marrow adipogenesis in diabetic complications. Adipocyte 3:263–272. doi:10.4161/adip.32215
Botolin S, Faugere M-C, Malluche H et al (2005) Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology 146:3622–3631. doi:10.1210/en.2004-1677
Ma Y-HV, Schwartz AV, Sigurdsson S et al (2014) Circulating sclerostin associated with vertebral bone marrow fat in older men but not women. J Clin Endocrinol Metab 99:E2584–E2590. doi:10.1210/jc.2013-4493
Krause MP, Riddell MC, Gordon CS et al (2009) Diabetic myopathy differs between Ins2Akita+/- and streptozotocin-induced Type 1 diabetic models. J Appl Physiol 106:1650–1659. doi:10.1152/japplphysiol.91565.2008
Jerković R, Bosnar A, Jurisić-Erzen D et al (2009) The effects of long-term experimental diabetes mellitus type I on skeletal muscle regeneration capacity. Coll Antropol 33:1115–1119
Kim TN, Park MS, Yang SJ et al (2010) Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 33:1497–1499. doi:10.2337/dc09-2310
Umegaki H (2015) Sarcopenia and diabetes: Hyperglycemia is a risk factor for age-associated muscle mass and functional reduction. J Diabetes Investig 6:623–624. doi:10.1111/jdi.12365
Macgilchrist C, Paul L, Ellis BM et al (2010) Lower-limb risk factors for falls in people with diabetes mellitus. Diabet Med 27:162–168. doi:10.1111/j.1464-5491.2009.02914.x
Colaianni G, Cuscito C, Mongelli T et al (2015) The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A 112:12157–12162. doi:10.1073/pnas.1516622112
Choi Y-K, Kim M-K, Bae KH et al (2013) Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract 100:96–101. doi:10.1016/j.diabres.2013.01.007
Palermo A, Strollo R, Maddaloni E et al (2014) Irisin is associated with osteoporotic fractures independently of bone mineral density, body composition or daily physical activity. Clin Endocrinol (Oxf). doi:10.1111/cen.12672
Acknowledgements
The authors thank Anda Naciu, MD, for the valuable support given to the drafting of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Andrea Palermo, Luca D’Onofrio, Raffaella Buzzetti, Silvia Manfrini and Nicola Napoli declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This is a systematic review and we did not perform any animal or human experiments for this work. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Palermo, A., D’Onofrio, L., Buzzetti, R. et al. Pathophysiology of Bone Fragility in Patients with Diabetes. Calcif Tissue Int 100, 122–132 (2017). https://doi.org/10.1007/s00223-016-0226-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0226-3