Abstract
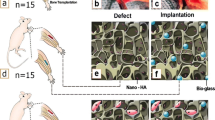
Large bone defects still represent a major burden in orthopedics, requiring bone-graft implantation to promote the bone repair. Along with autografts that currently represent the gold standard for complicated fracture repair, the bone tissue engineering offers a promising alternative strategy combining bone-graft substitutes with osteoprogenitor cells able to support the bone tissue ingrowth within the implant. Hence, the optimization of cell loading and distribution within osteoconductive scaffolds is mandatory to support a successful bone formation within the scaffold pores. With this purpose, we engineered constructs by seeding and culturing autologous, osteodifferentiated bone marrow mesenchymal stem cells within hydroxyapatite (HA)-based grafts by means of a perfusion bioreactor to enhance the in vivo implant-bone osseointegration in an ovine model. Specifically, we compared the engineered constructs in two different anatomical bone sites, tibia, and femur, compared with cell-free or static cell-loaded scaffolds. After 2 and 4 months, the bone formation and the scaffold osseointegration were assessed by micro-CT and histological analyses. The results demonstrated the capability of the acellular HA-based grafts to determine an implant-bone osseointegration similar to that of statically or dynamically cultured grafts. Our study demonstrated that the tibia is characterized by a lower bone repair capability compared to femur, in which the contribution of transplanted cells is not crucial to enhance the bone-implant osseointegration. Indeed, only in tibia, the dynamic cell-loaded implants performed slightly better than the cell-free or static cell-loaded grafts, indicating that this is a valid approach to sustain the bone deposition and osseointegration in disadvantaged anatomical sites.








Similar content being viewed by others
References
Sen MK, Miclau T (2007) Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury 38:75–80
Agarwal R, García AJ (2015) Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv Drug Deliv Rev. doi:10.1016/j.addr.2015.03.013
Kale AA, Cesare PE (1995) Osteoinductive agents. Basic science and clinical applications. Am J Orthop (Belle Mead NJ) 24(10):752–761
Drosos GI, Touzopoulos P, Ververidis A, Tilkeridis K, Kazakos K (2015) Use of demineralized bone matrix in the extremities. World J Orthop 6(2):269–277
Seebach C, Schulthesis J, Wilhelm K, Frank J, Henrich D (2010) Comparison of six bone-graft substitutes regarding to cell seeding efficiency, metabolism and growth behavior of human mesenchymal stem cells (MSC) in vitro. Injury 41:731–738
Petite H, Viateau V, Bensaïd W, Meunier A, de Pollak C, Bourguignon M, Oudina K, Sedel L, Guillemin G (2000) Tissue-engineered bone regeneration. Nat Biotechnol 18(9):959–963
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40(5):363–408
Gómez-Barrena E, Rosset P, Lozano D, Stanovici J, Ermthaller C, Gerbhard F (2015) Bone fracture healing: cell therapy in delayed unions and nonunions. Bone 70:93–101
Foldager C, Bendtsen M, Nygaard JV, Zou X, Bünger C (2009) Differences in early osteogenesis and bone micro-architecture in anterior lumbar interbody fusion with rhBMP-2, equine bone protein extract, and autograft. Bone 45(2):267–273
Mavrogenis AF, Dimitriou R, Parvizi J, Babis GC (2009) Biology of implant osseointegration. J Musculoskelet Neuronal Interact 9(2):61–71
Rosset P, Deschaseaux F, Layrolle P (2014) Cell therapy for bone repair. Orthop Traumatol Surg Res 100:S107–S112
Moretti M, Arrigoni C, Lovati AB, Talò G (2014) Bioinspired Cell Culture Bioreactors. In: Jabbari E (ed) Handbook of biomimetics and bioinspiration, 1st edn. Word Scientific, Singapore, pp 909–945
Komlev VS, Peyrin F, Mastrogiacomo M, Cedola A, Papadimitropoulos A, Rustichelli F, Cancedda R (2006) Kinetics of in vivo bone deposition by bone marrow stromal cells into porous calcium phosphate scaffolds: an X-ray computed microtomography study. Tissue Eng 12(12):3449–3458
Komlev VS, Mastrogiacomo M, Pereira RC, Peyrin F, Rustichelli F, Cancedda R (2010) Biodegradation of porous calcium phosphate scaffolds in an ectopic bone formation model studied by X-ray computed microtomograph. Eur Cell Mater 19:136–146
Tortelli F, Tasso R, Loiacono F, Cancedda R (2010) The development of tissue-engineered bone of different origin through endochondral and intramembranous ossification following the implantation of mesenchymal stem cells and osteoblasts in a murine model. Biomaterials 31(2):242–249
Marcacci M, Kon E, Zaffagnini S, Giardino R, Rocca M, Corsi A, Benvenuti A, Bianco P, Quarto R, Martin I, Muraglia A, Cancedda R (1999) Reconstruction of extensive long-bone defects in sheep using porous hydroxyapatite sponges. Calcif Tissue Int 64(1):83–90
Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M, Cancedda R (2007) Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng 13(5):947–955
Güven S, Mehrkens A, Saxer F, Schaefer DJ, Martinetti R, Martin I, Scherberich A (2011) Engineering of large osteogenic grafts with rapid engraftment capacity using mesenchymal and endothelial progenitors from human adipose tissue. Biomaterials 32(25):5801–5809
Timmins NE, Scherberich A, Früh JA, Heberer M, Martin I, Jakob M (2007) Three-dimensional cell culture and tissue engineering in a T-CUP (tissue culture under perfusion). Tissue Eng 13(8):2021–2028
Braccini A, Wendt D, Jaquiery C, Jakob M, Heberer M, Kenins L, Wodnar-Filipowicz A, Quarto R, Martin I (2005) Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells 23(8):1066–1072
Cheng M, Moretti M, Engelmayr GC, Freed LE (2009) Insulin-like growth factor-I and slow, bi-directional perfusion enhance the formation of tissue-engineered cardiac grafts. Tissue Eng Part A 15:645–653
Valonen PK, Moutos FT, Kusanagi A, Moretti MG, Diekman BO, Welter JF, Caplan AI, Guilak F, Freed LE (2010) In vitro generation of mechanically functional cartilage grafts based on adult human stem cells and 3D-woven poly(epsilon-caprolactone) scaffolds. Biomaterials 31(8):2193–2200
Nuss K, Auer JA, Boos A, von Rechenberg B (2006) An animal model in sheep for biocompatibility testing of biomaterials in cancellous bones. BMC Musculoskelet Disord 7:67
Potes JC, da Costa Reis J, Capela e Silva F, Relvas C, Cabrita AS, Simoes JA (2008) The sheep as an animal model in orthopaedic research. Exp Pathol Health Sci 2(1):29–32
Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG (2007) Animal models for implant biomaterial research in bone: a review. Eur Cell Mater 13:1–10
Patel N, Brooks RA, Clarke MT, Lee PM, Rushton N, Gibson IR, Best SM, Bonfield W (2005) In vivo assessment of hydroxyapatite and silicate-substituted hydroxyapatite granules using an ovine defect model. J Mater Sci Mater Med 16(5):429–440
Schneider OD, Mohn D, Fuhrer R, Klein K, Kämpf K, Nuss KM, Sidler M, Zlinszky K, von Rechenberg B, Stark WJ (2011) Biocompatibility and bone formation of flexible, cotton wool-like PLGA/Calcium Phosphate nanocomposites in sheep. Open Orthop J 5:63–71
Han D, Han N, Xue F, Zhang P (2015) A novel specialized staging system for cancellous fracture healing, distinct from traditional healing pattern of diaphysis cortical fracture? Int J Clin Exp Med 8(1):1301–1304
Le Guehennec L, Goyenvalle E, Aguado E, Houchmand-Cuny M, Enkel B, Pilet P, Daculsi G, Layrolle P (2005) Small-animal models for testing macroporous ceramic bone substitutes. J Biomed Mater Res B Appl Biomater 72(1):69–78
An YH, Friedman RJ (1999) Animal models in orthopaedic research. CRC Press, Boca Raton
Mastrogiacomo M, Scaglione S, Martinetti R, Dolcini L, Beltrame F, Cancedda R, Quarto R (2006) Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials 27:3230–3237
Bersini S, Gilardi M, Arrigoni C, Talò G, Zamai M, Zagra L, Caiolfa V, Moretti M (2016) Human in vitro 3D co-culture model to engineer vascularized bone-mimicking tissues combining computational tools and statistical experimental approach. Biomaterials 76:157–172
Bottagisio M, Lovati AB, Lopa S, Moretti M (2015) Osteogenic differentiation of human and ovine bone marrow stromal cells in response to β-glycerophosphate and monosodium phosphate. Cell Reprogr 17(4):235–242
Lopa S, Colombini A, Stanco D, de Girolamo L, Sansone V, Moretti M (2014) Donor-matched mesenchymal stem cells from knee infrapatellar and subcutaneous adipose tissue of osteoarthritic donors display differential chondrogenic and osteogenic commitment. Eur Cell Mater 27:298–311
Martini L, Fini M, Giavaresi G, Giardino R (2001) Sheep model in orthopedic research: a literature review. Comp Med 51:292–299
Gardel LS, Serra LA, Reis RL, Gomes ME (2014) Use of perfusion bioreactors and large animal models for long bone tissue engineering. Tissue Eng Part B Rev 20(2):126–146
Nafei A, Danielsen CC, Linde F, Hvid I (2000) Properties of growing trabecular ovine bone. Part I: mechanical and physical properties. J Bone Joint Surg Br 82:910–920
Nafei A, Kabel J, Odgaard A, Linde F, Hvid I (2000) Properties of growing trabecular ovine bone. Part II: architectural and mechanical properties. J Bone Joint Surg Br 82:921–927
Reichert JC, Woodruff MA, Friis T, Quent VM, Gronthos S, Duda GN, Schütz MA, Hutmacher DW (2010) Ovine bone- and marrow-derived progenitor cells and their potential for scaffold-based bone tissue engineering applications in vitro and in vivo. J Tissue Eng Regen Med 4:565–576
Ding M, Odgaard A, Linde F, Hvid I (2002) Age-related variations in the microstructure of human tibial cancellous bone. J Orthop Res 20:615–621
Ludwig SC, Kowalski JM, Boden SD (2000) Osteoinductive bone graft substitutes. Eur Spine J 9(1):119–125
Hench LL (1998) Biomaterials: a forecast for the future. Biomaterials 19:1419–1423
Demontiero O, Vidal C, Duque G (2012) Aging and bone loss: new insights for the clinician. Ther Adv Musculoskelet Dis 4(2):61–76
Dias GJ, Mahoney P, Swain M, Kelly RJ, Smith RA, Ali MA (2010) Keratin-hydroxyapatite composites: biocompatibility, osseointegration, and physical properties in an ovine model. J Biomed Mater Res A 95A(4):1084–1095
Kitoh H, Kawasumi M, Kaneko H, Ishiguro N (2009) Differential effects of culture-expanded bone marrow cells on the regeneration of bone between the femoral and the tibial lengthening. J Pediatr Orthop 29(6):643–649
Emara KM, Diab RA, Emara AK (2015) Recent biological trends in management of fracture non-union. World J Orthop 6(8):623–628
Chistolini P, Ruspantini I, Bianco P, Corsi A, Cancedda R, Quarto R (1999) Biomechanical evaluation of cell-loaded and cell-free hydroxyapatite implants for the reconstruction of segmental bone defects. J Mater Sci Mater Med 10(12):739–742
Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, Boyde A, Ruspantini I, Chistolini P, Rocca M, Giardino R, Cancedda R, Quarto R (2000) Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res 49(3):328–337
Khan WS, Rayan F, Dhinsa BS, Marsh D (2012) An osteoconductive, osteoinductive, and osteogenic tissue-engineered product for trauma and orthopaedic surgery: how far are we? Stem Cells Int. doi:10.1155/2012/236231
Acknowledgments
This work was funded by Regione Lombardia with POR FESR 2007-2013 resources (Grant ID: ATP 2009, No. 13396272). The authors thank Finceramica for providing the Engipore® scaffolds.
Author contribution
Guarantor: author#9. Study concept and design: authors #1, #2, #3, #4, #8 and #9. Data acquisition and analyses: authors #1, #2, #3, #4, #5 and #7. Data interpretation: authors #1, #2, #3, #4 and #6. Drafting of the manuscript: authors #1, #2, #3, #4, #5, #6 and #7. Critical revision of the manuscript for important intellectual contents: authors #1, #2, #3, #6, #8 and #9. All authors approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M Moretti has received research grant from Regione Lombardia with POR FESR 2007–2013 resources (Grant ID: ATP 2009, No. 13396272) and he is inventor of the patent #WO2008098165A2 issued by USPTO.
Human and Animal Rights and Informed Consent
This article does not contain studies with human participants performed by any of the authors. All applicable international, national and/or institutional guidelines for the care and use of animals were followed. Specifically, the Lazzaro Spallanzani Institute Animal Care and Use Committee (IACUC) approved the whole study. Animals and their care were handled in compliance with institutional guidelines as defined in national (Law 116/92, Authorization n.19/2008-A issued March 6, 2008, by the Italian Ministry of Health) and international laws and policies (EEC Council Directive 86/609, OJ L 358. 1, December 12, 1987; Standards for the Care and Use of Laboratory Animals—UCLA, U. S. National Research Council, Statement of Compliance A5023-01, November 6, 1998). The animals were housed at the facilities of the Lazzaro Spallanzani Institute according international standards. A certified veterinarian—responsible for health monitoring, animal welfare supervision, experimental protocols and procedure revision—regularly checked animals. All procedures on animals performed in this study were in accordance with the ethical standards.
Additional information
S. Lopa and C. Recordati have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lovati, A.B., Lopa, S., Recordati, C. et al. In Vivo Bone Formation Within Engineered Hydroxyapatite Scaffolds in a Sheep Model. Calcif Tissue Int 99, 209–223 (2016). https://doi.org/10.1007/s00223-016-0140-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0140-8