Abstract
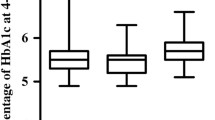
The pathogenesis of diabetic osteopenia is unclear. The markers of bone metabolism may show some changes in diabetic patients. In this study, we investigated the effect of glycemic control on serum osteocalcin level and urinary hydroxyproline excretion and the relations of these markers to duration of diabetes, C-peptide status, and body mass index. Twenty-seven men with poorly controlled diabetes mellitus (DM) (HbA1>9%, fasting plasma glucose>7.8 mmol/liter) between ages 25 and 60 years (means±SD 46.6±10.4) were included in the study. Duration of diabetes was 5.8±4.7 years, body mass index (BMI) was 25.9±3.5 kg/m2, and fasting C-peptide was 2.33 (1.05–3.21) μg/liter. None of the patients had a disease or were treated with drugs that would interfer with calcium or phosphate metabolism and/or bone structure. They were free from chronic diabetic complications. Of these patients, 11 were lost to follow-up before metabolic control was achieved. The remaining 16 patients obtained good glycemic control (HbA1<8.3%, fasting plasma glucose <7.8 mmol/liter) and completed the study. Serum osteocalcin level and urinary hydroxyproline eceretion were determined before and after glycemic control. Urinary hydroxyproline excretion was not significantly changed by glycemic control [17.8 (7.1–23.2) versus 18.1 (10.9–28.1) mg/m2 day, P>0.05]. However, serum osteocalein level was significantly elevated (5.04±1.43 versus 4.17±1.83 μg/liter, P=0.04). We found no correlation among fasting plasma glucose, HbA1, and fasting serum C-peptide levels with urinary hydroxyproline excretion. There was also no correlation between serum osteocalcin and fasting plasma glucose or serum C-peptide, but HbA1 was negatively correlated with serum osteocalcin (P=0.01). No correlation was found between DM duration and BMI in the patients with serum osteocalcin level and urinary hydroxyproline excretion. To eliminate the possible effect of exogenous insulin on bone metabolism, the correlation analysis between the markers and C-peptide was further repeated in oral agents-treated patients. Serum C-peptide was not correlated to serum osteocalcin or urinary hydroxyproline in this subgroup of patients. Knowing that serum osteocalcin is a marker of bone formation, we concluded that osteoblast function may improve by glycemic control in diabetic patients; this may be due to correction of metabolic abnormalities associated with insulinopenia.
Similar content being viewed by others
References
Levin ME, Boisseau VC, Avioli LV (1976) Effect of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. N Engl J Med 294:241–245
McNair P, Madsbad S, Christiansen C, Faber OK, Transbol I, Binder C (1978) Osteopenia in insulin-treated diabetes mellitus: its relation to age at onset, sex and duration of disease. Diabetologia 15:87–90
Auwerx J, Dequer J, Bouillon R, Geusens P, Nijs J (1988) Mineral metabolism and bone mass at peripheral and axial skeleton in diabetes mellitus. Diabetes 37:8–12
Mathiassen B, Nielsen S, Ditzel J, Rodbro P (1990) Long-term bone loss in insulin-dependent diabetes mellitus. J Intern Med 227:325–327
Ishida H, Seino Y, Matsukura S, Ikeda M, Yawata M, Yamashita G, Ishizuka S, Imura H (1985) Diabetic osteopenia and circulating levels of vitamin D metabolites in type 2 (noninsulin-dependent) diabetes. Metabolism 34:797–801
DeLeeuw I, Abs R (1977) Bone mass and bone density in maturity type diabetes measured by the 125I photon-absorption technique. Diabetes 26:1130–1135
Barrett-Connor E, Holbrook TL (1992) Sex differences in osteoporosis in older adults with non-insulin-dependent diabetes mellitus. JAMA 268:3333–3337
Schneider LE, Schedl H (1972) Diabetes and intestinal calcium absorption in the rat. Am J Physiol 223:1319–1323
Schneider LE, Schedl HP, McCain T, Haussler MR (1977) Experimental diabetes reduces circulating 1, 25 dihydroxy vitamin D in the rat. Science 196:1452–1454
Raskin P, Stevenson MRM, Barilla DE, Pak CYC (1978) The hypercalciuria of diabetes mellitus: its amelioration with insulin. Clin Endocrinol 9:329–335
Schedl HP, Health III H, Wenger J (1978) Serum calcitonin and parathyroid hormone in experimental diabetes: effects of insulin treatment. Endocrinology 103:1368–1373
Wientroub S, Eisenberg D, Tardiman R, Weissman SL, Salama R (1980) Is diabetic osteoporosis due to microangiopathy? Lancet 2:983
Minne HW, Pfeilschifter J, Scharla SH, Mutschelknauss S, Schwarz A, Krempien B, Ziegler R (1984) Inflammationmediated osteopenia in the rat: a new animal model for pathological loss of bone mass. Endocrinology 115:50–54
Ziegler R (1992) Diabetes mellitus and bone metabolism. Horm Metab Res (suppl) 26:90–94
Delmas PD (1991) Biochemical markers of bone turnover. methodology and clinical use in osteoporosis. Am J Med (suppl 5B):59S-63S
Glajchen N, Epstein S, Ismail F, Fallon TM, Chakrabarti S (1988) Bone mineral metabolism in experimental diabetes mellitus: osteocalcin as a measure of bone remodeling. Endocrinology 123:290–295
Ishida H, Seino Y, Taminato T, Usami M, Takeshita N, Seino Y, Tsutsumi C, Moriuchi S, Akiyama Y, Hara K, Imura H (1988) Circulating levels and bone contents of bone γ-carboxy-glutamic acid-containing protein are decreased in streptozocin-induced diabetes. Possible marker for diabetic osteopenia. Diabetes 37:702–706
Verhaeghe J, VanHerck E, Visser WJ, Suiker AMH, Thomasset M, Einhorn TA, Faierman E, Boillon R (1990) Bone and mineral metabolism in BB rats with long-term diabetes. Decreased bone turnover and osteoporosis. Diabetes 39:477–482
Hough S, Avioli LV, Bergfeld MA, Fallon MD, Slatopolsky E, Teitelbaum SL (1981) Correction of abnormal bone and mineral metabolism in chronic streptozotocin-induced diabetes mellitus in the rat by insulin therapy. Endocrinology 108:2228–2234
Marrikakis ME, Karli J, Antoniades LG, Sfikakis PP, Kontoyannis SA, Moulopoulou DS, Koutras DA, Raptis SA (1993) Twenty-four hours urinary hydroxyproline excretion in long-term experimental diabetes mellitus. Horm Metab Res 25:498–499
Cantini F, Arcangeli A, Bellandi F, Pedone T, Villani G, Ponzio A, Palchetti R (1992) Serum osteocalcin and diabetes mellitus. A study of 98 patients. Minerva Med 83:129–133
Guarneri MP, Weber G, Gallia P, Chiumello G (1993) Effect of insulin treatment on osteocalcin levels in diabetic children and adolescents. J Endocrinol Invest 16:505–509
Ewing DJ, Clarke BF (1982) Diagnosis and management of diabetic autonomic neuropathy. Br Med J 285:916–918
Hernberg CA (1952) The bone structure in alloxan-induced diabetes mellitus in rats. Acta Med Scand 142:274–277
Hadjidakis D, Lempert UG, Minne HW, Ziegler R (1993) Bone loss in experimental diabetes. Comparison with the model of inflammation-mediated osteopenia. Horm Metab Res 25:77–81
Wakasugi M, Wakao R, Tawata M, Gan N, Koizumi K, Onaya T (1993) Bone mineral density measured by dual energy X-ray absorptiometry in patients with non-insulin-dependent diabetes mellitus. Bone 14:29–33
Heath H III, Lambert PW, Service FJ, Arnaud SB (1979) Calcium homeostasis in diabetes mellitus. J Clin Endocrinol Metab 49:462–466
Weiss RE, Reddi AH (1980) Influence of experimental diabetes and insulin on matrix-induced cartilage and bone differentiation. Am J Physiol 238 (Endocrinol Metab):E200-E207
Reid IR, Evans MC, Cooper GJS, Ames RW, Stapleton J (1993) Circulating insulin levels are related to bone density in normal postmenopausal women. Am J Physiol 265 (Endocrinol Metab 28):E655-E659
McNair P, Madsbad S, Christiansen C, Christensen MS, Faber OK, Binder C, Transbol I (1979) Bone loss in diabetes: effects of metabolic state. Diabetologia 17:283–286
Goodman WG, Hori MT (1984) Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization. Diabetes 33:825–831
Levy JR, Murray E, Manolagas S, Olefsky JM (1986) Demonstration of insulin receptors and modulation of alkaline phosphatase activity by insulin in rat osteoblastic cells. Endocrinology 119:1786–1792
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sayinalp, S., Gedik, O. & Koray, Z. Increasing serum osteocalcin after glycemic control in diabetic men. Calcif Tissue Int 57, 422–425 (1995). https://doi.org/10.1007/BF00301944
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00301944