Abstract
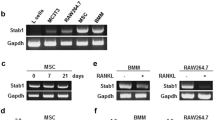
Genetic studies in human and mice have pinpointed an essential role of Notch signaling in osteoblast and osteoclast differentiation during skeletal development and bone remodeling. However, the factors and pathways regulating Notch activation in bone cells remain largely unknown. In this in vitro study, we have provided evidence that two of the TspanC8 subfamily members of tetraspanins, Tspan-5 and Tspan-10, are up-regulated during osteoclast differentiation and knockdown of their expression by shRNAs dramatically inhibits osteoclastogenesis. Loss of Tspan-5 and Tspan-10 in osteoclast lineage cells results in attenuation of ADAM10 maturation and Notch activation. Therefore, these two tetraspanins play a critical role in osteoclast formation, at least in part, by modulating Notch signaling pathway.





Similar content being viewed by others
References
Crockett JC, Rogers MJ, Coxon FP, Hocking LJ, Helfrich MH (2011) Bone remodelling at a glance. J Cell Sci 124:991–998
Lazner F, Gowen M, Pavasovic D, Kola I (1999) Osteopetrosis and osteoporosis: two sides of the same coin. Hum Mol Genet 8:1839–1846
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Teitelbaum SL, Ross FP (2003) Genetic regulation of osteoclast development and function. Nat Rev Genet 4:638–649
Nakashima T, Hayashi M, Takayanagi H (2012) New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab: TEM 23:582–590
Engin F, Lee B (2010) NOTCHing the bone: insights into multi-functionality. Bone 46:274–280
Regan J, Long F (2013) Notch signaling and bone remodeling. Curr Osteoporos Rep 11:126–129
Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137:216–233
Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F, Ross FP, Teitelbaum SL (2008) NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem 283:6509–6518
Fukushima H, Nakao A, Okamoto F, Shin M, Kajiya H, Sakano S, Bigas A, Jimi E, Okabe K (2008) The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis. Mol Cell Biol 28:6402–6412
Simpson MA, Irving MD, Asilmaz E, Gray MJ, Dafou D, Elmslie FV, Mansour S, Holder SE, Brain CE, Burton BK, Kim KH, Pauli RM, Aftimos S, Stewart H, Kim CA, Holder-Espinasse M, Robertson SP, Drake WM, Trembath RC (2011) Mutations in NOTCH2 cause Hajdu–Cheney syndrome, a disorder of severe and progressive bone loss. Nat Genet 43:303–305
Christian LM (2012) The ADAM family: insights into Notch proteolysis. Fly 6:30–34
Hori K, Sen A, Artavanis-Tsakonas S (2013) Notch signaling at a glance. J Cell Sci 126:2135–2140
Hemler ME (2005) Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 6:801–811
Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F (2009) Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol 19:434–446
Hemler ME (2014) Tetraspanin proteins promote multiple cancer stages. Nat Rev Cancer 14:49–60
Bailey RL, Herbert JM, Khan K, Heath VL, Bicknell R, Tomlinson MG (2011) The emerging role of tetraspanin microdomains on endothelial cells. Biochem Soc Trans 39:1667–1673
Dornier E, Coumailleau F, Ottavi JF, Moretti J, Boucheix C, Mauduit P, Schweisguth F, Rubinstein E (2012) TspanC8 tetraspanins regulate ADAM10/Kuzbanian trafficking and promote Notch activation in flies and mammals. J Cell Biol 199:481–496
Dunn CD, Sulis ML, Ferrando AA, Greenwald I (2010) A conserved tetraspanin subfamily promotes Notch signaling in Caenorhabditis elegans and in human cells. Proc Natl Acad Sci USA 107:5907–5912
Haining EJ, Yang J, Bailey RL, Khan K, Collier R, Tsai S, Watson SP, Frampton J, Garcia P, Tomlinson MG (2012) The TspanC8 subgroup of tetraspanins interacts with A disintegrin and metalloprotease 10 (ADAM10) and regulates its maturation and cell surface expression. J Biol Chem 287:39753–39765
Ishii M, Iwai K, Koike M, Ohshima S, Kudo-Tanaka E, Ishii T, Mima T, Katada Y, Miyatake K, Uchiyama Y, Saeki Y (2006) RANKL-induced expression of tetraspanin CD9 in lipid raft membrane microdomain is essential for cell fusion during osteoclastogenesis. J Bone Miner Res 21:965–976
Takeda Y, Tachibana I, Miyado K, Kobayashi M, Miyazaki T, Funakoshi T, Kimura H, Yamane H, Saito Y, Goto H, Yoneda T, Yoshida M, Kumagai T, Osaki T, Hayashi S, Kawase I, Mekada E (2003) Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J Cell Biol 161:945–956
Iwai K, Ishii M, Ohshima S, Miyatake K, Saeki Y (2007) Expression and function of transmembrane-4 superfamily (tetraspanin) proteins in osteoclasts: reciprocal roles of Tspan-5 and NET-6 during osteoclastogenesis. Allergol Int 56:457–463
Zhou J, Ye S, Fujiwara T, Manolagas SC, Zhao H (2013) Steap4 plays a critical role in osteoclastogenesis in vitro by regulating cellular iron/reactive oxygen species (ROS) levels and cAMP response element-binding protein (CREB) activation. J Biol Chem 288:30064–30074
Takeshita S, Kaji K, Kudo A (2000) Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res 15:1477–1488
Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC (1992) Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science (New York, N.Y.) 257:88–91
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Ye S, Fowler TW, Pavlos NJ, Ng PY, Liang K, Feng Y, Zheng M, Kurten R, Manolagas SC, Zhao H (2011) LIS1 regulates osteoclast formation and function through its interactions with dynein/dynactin and Plekhm1. PLoS ONE 6:e27285
Verrier S, Hogan A, McKie N, Horton M (2004) ADAM gene expression and regulation during human osteoclast formation. Bone 35:34–46
Canalis E, Parker K, Feng JQ, Zanotti S (2013) Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology 154:623–634
Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F (2008) Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med 14:306–314
Yamada T, Yamazaki H, Yamane T, Yoshino M, Okuyama H, Tsuneto M, Kurino T, Hayashi S, Sakano S (2003) Regulation of osteoclast development by Notch signaling directed to osteoclast precursors and through stromal cells. Blood 101:2227–2234
Sethi N, Dai X, Winter CG, Kang Y (2011) Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19:192–205
Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE, Boyce BF, Lee B (2008) Dimorphic effects of Notch signaling in bone homeostasis. Nat Med 14:299–305
Acknowledgments
The authors would like to thank Drs. Stavros C Manolagas and Charles A Obrien for their critic of the manuscript prior to submission and Erin Hogan for her support in microscopic analysis. This work was supported by National Institutes of Health Grants AR062012 and P01 AG13918. This work was also supported by the University of Arkansas for Medical Sciences Tobacco Settlement Funds provided by the Arkansas Biosciences Institute.
Human and Animal Rights and Informed Consent
The usage and handling of mice were approved by UAMS Institutional Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jian Zhou and Toshifumi Fujiwara authors have contributed equally to this study.
The authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, J., Fujiwara, T., Ye, S. et al. Downregulation of Notch Modulators, Tetraspanin 5 and 10, Inhibits Osteoclastogenesis in Vitro. Calcif Tissue Int 95, 209–217 (2014). https://doi.org/10.1007/s00223-014-9883-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9883-2