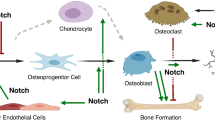
Abstract
Notch signaling plays context-dependent roles in the development and maintenance of many cell types and tissues in mammals. In the skeleton, both osteoblasts and osteoclasts require Notch signaling for proper differentiation and function, and the specific roles of Notch are dependent on the differentiation status of the cell. The recent discovery of activating NOTCH2 mutations as the cause of Hajdu-Cheney syndrome has highlighted the significance of Notch signaling in human bone physiology.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33.
Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–47.
Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–612.
Canalis E. Notch signaling in osteoblasts. Sci Signal. 2008;1:17.
Deregowski V, Gazzerro E, Priest L, Rydziel S, Canalis E. Notch 1 overexpression inhibits osteoblastogenesis by suppressing Wnt/beta-catenin but not bone morphogenetic protein signaling. J Biol Chem. 2006;281:6203–10.
• Sciaudone M, Gazzerro E, Priest L, Delany AM, Canalis E. Notch 1 impairs osteoblastic cell differentiation. Endocrinology. 2003;144:5631–9. An early in vitro study demonstrating a suppresive role for Notch signaling in osteoblast differentiation.
Tezuka K, Yasuda M, Watanabe N, et al. Stimulation of osteoblastic cell differentiation by Notch. J Bone Miner Res. 2002;17:231–9.
Nobta M, Tsukazaki T, Shibata Y, et al. Critical regulation of bone morphogenetic protein-induced osteoblastic differentiation by Delta1/Jagged1-activated Notch1 signaling. J Biol Chem. 2005;280:15842–8.
Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–39.
Wong PC, Zheng H, Chen H, et al. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–92.
Dunwoodie SL, Clements M, Sparrow DB, Sa X, Conlon RA, Beddington RS. Axial skeletal defects caused by mutation in the spondylocostal dysplasia/pudgy gene Dll3 are associated with disruption of the segmentation clock within the presomitic mesoderm. Development. 2002;129:1795–806.
• Hilton MJ, Tu X, Wu X, et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306–14. This mouse genetic study establishes that physiological Notch singaling suppresses bone formation in vivo.
• Tu X, Chen J, Lim J, et al. Physiological notch signaling maintains bone homeostasis via RBPjk and Hey upstream of NFATc1. PLoS Genet. 2012;8:e1002577. This study delineates the mechanism through which physiological Notch signaling suppresses bone formation, and identifies Notch2 as a critical regulator.
Salie R, Kneissel M, Vukevic M, et al. Ubiquitous overexpression of Hey1 transcription factor leads to osteopenia and chondrocyte hypertrophy in bone. Bone. 2010;46:680–94.
Engin F, Yao Z, Yang T, et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14:299–305.
• Tao J, Chen S, Yang T, et al. Osteosclerosis owing to Notch gain of function is solely Rbpj-dependent. J Bone Miner Res. 2010;25:2175–83. These two mouse genetic studies demonstrate that hyperactivation of Notch signaling through RBPj impairs bone homeostasis.
Zanotti S, Smerdel-Ramoya A, Stadmeyer L, Durant D, Radtke F, Canalis E. Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology. 2008;149:3890–9.
Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–5.
• Canalis E, Parker K, Feng JQ, Zanotti S. Osteoblast lineage-specific effects of Notch activation in the skeleton. Endocrinology. 2013;154(2):623–34. This study highlights the stage-specific effects of hyperactive Notch signaling in the osteoblast lineage.
Canalis E, Parker K, Feng JQ, Zanotti S. Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology. 2013;154:623–34.
Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Annu Rev Pathol. 2008;3:457–84.
• Bai S, Kopan R, Zou W, et al. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem. 2008;283:6509–18. This study demonstrates both direct and osteoblast-mediated regulation of osteoclastogenesis by Notch.
Yamada T, Yamazaki H, Yamane T, et al. Regulation of osteoclast development by Notch signaling directed to osteoclast precursors and through stromal cells. Blood. 2003;101:2227–34.
Fukushima H, Nakao A, Okamoto F, et al. The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis. Mol Cell Biol. 2008;28:6402–12.
Sekine C, Koyanagi A, Koyama N, Hozumi K, Chiba S, Yagita H. Differential regulation of osteoclastogenesis by Notch2/Delta-like 1 and Notch1/Jagged1 axes. Arthritis Res Ther. 2012;14:R45.
Brennan AM, Pauli RM. Hajdu-Cheney syndrome: evolution of phenotype and clinical problems. Am J Med Genet. 2001;100:292–310.
Isidor B, Lindenbaum P, Pichon O, et al. Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis. Nat Genet. 2011;43:306–8.
Simpson MA, Irving MD, Asilmaz E, et al. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat Genet. 2011;43:303–5.
• Majewski J, Schwartzentruber JA, Caqueret A, et al. Mutations in NOTCH2 in families with Hajdu-Cheney syndrome. Hum Mutat. 2011;32:1114–7. These studies identify NOTCH2 mutations as the cause for Hajdu-Cheney Syndrome.
Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–71.
Isidor B, Le Merrer M, Exner GU, et al. Serpentine fibula-polycystic kidney syndrome caused by truncating mutations in NOTCH2. Hum Mutat. 2011;32:1239–42.
Gray MJ, Kim CA, Bertola DR, et al. Serpentine fibula polycystic kidney syndrome is part of the phenotypic spectrum of Hajdu-Cheney syndrome. Eur J Hum Genet. 2012;20:122–4.
Turnpenny PD, Ellard S. Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2012;20:251–7.
Oda T, Elkahloun AG, Pike BL, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–42.
Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–51.
• McDaniell R, Warthen DM, Sanchez-Lara PA, et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79:169–73. These studies discovered the role of impaired Notch signaling in Alagille syndrome.
Sanderson E, Newman V, Haigh SF, Baker A, Sidhu PS. Vertebral anomalies in children with Alagille syndrome: an analysis of 50 consecutive patients. Pediatr Radiol. 2002;32:114–9.
Berrocal T, Gamo E, Navalón J, et al. Syndrome of Alagille: radiological and sonographic findings. A review of 37 cases. Eur Radiol. 1997;7:115–8.
Krantz ID, Piccoli DA, Spinner NB. Alagille syndrome. J Med Genet. 1997;34:152–7.
Hoffenberg EJ, Narkewicz MR, Sondheimer JM, Smith DJ, Silverman A, Sokol RJ. Outcome of syndromic paucity of interlobular bile ducts (Alagille syndrome) with onset of cholestasis in infancy. J Pediatr. 1995;127:220–4.
Bales CB, Kamath BM, Munoz PS, et al. Pathologic lower extremity fractures in children with Alagille syndrome. J Pediatr Gastroenterol Nutr. 2010;51:66–70.
• Kung AW, Xiao SM, Cherny S, et al. Association of JAG1 with bone mineral density and osteoporotic fractures: a genome-wide association study and follow-up replication studies. Am J Hum Genet. 2010;86:229–39. This study Links JAG1 polymorphism with bone mineral density in a diverse human population.
Acknowledgements
Work in the Long lab is supported by NIH grant AR055923. J. Regan is a postdoctoral fellow supported by NIH T32 HL007873.
Disclosure
J. Regan declares no conflicts of interest. F. Long declares no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Regan, J., Long, F. Notch Signaling and Bone Remodeling. Curr Osteoporos Rep 11, 126–129 (2013). https://doi.org/10.1007/s11914-013-0145-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-013-0145-4