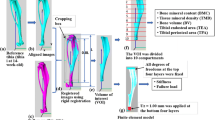
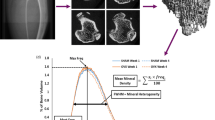
Abstract
Recently, it has been shown that transient bone biology can be observed in vivo using time-lapse micro-computed tomography (μCT) in the mouse tail bone. Nevertheless, in order for the mouse tail bone to be a model for human disease, the hallmarks of any disease must be mimicked. The aim of this study was to investigate whether postmenopausal osteoporosis could be modeled in caudal vertebrae of C57Bl/6 mice, considering static and dynamic bone morphometry as well as mechanical properties, and to describe temporal changes in bone remodeling rates. Twenty C57Bl/6 mice were ovariectomized (OVX, n = 11) or sham-operated (SHM, n = 9) and monitored with in vivo μCT on the day of surgery and every 2 weeks after, up to 12 weeks. There was a significant decrease in bone volume fraction for OVX (−35%) compared to SHM (+16%) in trabecular bone (P < 0.001). For OVX, high-turnover bone loss was observed, with the bone resorption rate exceeding the bone formation rate (P < 0.001). Furthermore there was a significant decrease in whole-bone stiffness for OVX (−16%) compared to SHM (+11%, P < 0.001). From these results we conclude that the mouse tail vertebra mimics postmenopausal bone loss with respect to these parameters and therefore might be a suitable model for postmenopausal osteoporosis. When evaluating temporal changes in remodeling rates, we found that OVX caused an immediate increase in bone resorption rate (P < 0.001) and a delayed increase in bone formation rate (P < 0.001). Monitoring transient bone biology is a promising method for future research.





Similar content being viewed by others
References
Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jönsson B (2011) Osteoporosis: burden, health care provision and opportunities in the EU. Arch Osteoporos. doi:10.1007/s11657-011-0060-1
Reinwald S, Burr D (2008) Review of nonprimate, large animal models for osteoporosis research. J Bone Miner Res 23:1353–1368
Kimmel DB (1996) Animal models for in vivo experimentation in osteoporosis research. In: Marcus R, Feldman D, Kelsey J (eds) Osteoporosis. Academic Press, San Diego, pp 671–690
Kalu DN (1991) The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15:175–191
Brouwers JEM, Lambers FM, Gasser JA, van Rietbergen B, Huiskes R (2008) Bone degeneration and recovery after early and late bisphosphonate treatment of ovariectomized Wistar rats assessed by in vivo micro-computed tomography. Calcif Tissue Int 82:202–211
Boyd SK, Davison P, Müller R, Gasser JA (2006) Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone 39:854–862
Waarsing JH, Day JS, van der Linden JC, Ederveen AG, Spanjers C, De Clerck N, Sasov A, Verhaar JA, Weinans H (2004) Detecting and tracking local changes in the tibiae of individual rats: a novel method to analyse longitudinal in vivo micro-CT data. Bone 34:163–169
Wronski TJ, Dann LM, Scott KS, Cintron M (1989) Long-term effects of ovariectomy and aging on the rat skeleton. Calcif Tissue Int 45:360–366
Tian XY, Liu XQ, Chen HY, Setterberg RB, Li M, Jee WS (2008) Greater efficacy of alfacalcidol in the red than in the yellow marrow skeletal sites in adult female rats. J Musculoskelet Neuronal Interact 8:257–266
Wronski TJ, Dann LM, Horner SL (1989) Time course of vertebral osteopenia in ovariectomized rats. Bone 10:295–301
Miyakoshi N, Sato K, Abe T, Tsuchida T, Tamura Y, Kudo T (1999) Histomorphometric evaluation of the effects of ovariectomy on bone turnover in rat caudal vertebrae. Calcif Tissue Int 64:318–324
Li M, Shen Y, Qi H, Wronski TJ (1996) Comparative study of skeletal response to estrogen depletion at red and yellow marrow sites in rats. Anat Rec 245:472–480
Klein RF (2008) Genetics of osteoporosis—utility of mouse models. J Musculoskelet Neuronal Interact 8:287–290
Alexander JM, Bab I, Fish S, Müller R, Uchiyama T, Gronowicz G, Nahounou M, Zhao Q, White DW, Chorev M, Gazit D, Rosenblatt M (2001) Human parathyroid hormone 1–34 reverses bone loss in ovariectomized mice. J Bone Miner Res 16:1665–1673
Cano A, Dapia S, Noguera I, Pineda B, Hermenegildo C, del Val R, Caeiro JR, Garcia-Perez MA (2008) Comparative effects of 17beta-estradiol, raloxifene and genistein on bone 3D microarchitecture and volumetric bone mineral density in the ovariectomized mice. Osteoporos Int 19:793–800
Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG (2005) Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner Res 20:1085–1092
Li CY, Schaffler MB, Wolde-Semait HT, Hernandez CJ, Jepsen KJ (2005) Genetic background influences cortical bone response to ovariectomy. J Bone Miner Res 20:2150–2158
Iwaniec UT, Yuan D, Power RA, Wronski TJ (2006) Strain-dependent variations in the response of cancellous bone to ovariectomy in mice. J Bone Miner Res 21:1068–1074
Klinck J, Boyd SK (2008) The magnitude and rate of bone loss in ovariectomized mice differs among inbred strains as determined by longitudinal in vivo micro-computed tomography. Calcif Tissue Int 83:70–79
Webster D, Wasserman E, Ehrbar M, Weber F, Bab I, Müller R (2010) Mechanical loading of mouse caudal vertebrae increases trabecular and cortical bone mass-dependence on dose and genotype. Biomech Model Mechanobiol 9:737–747
Lambers FM, Schulte FA, Kuhn G, Webster DJ, Muller R (2011) Mouse tail vertebrae adapt to cyclic mechanical loading by increasing bone formation rate and decreasing bone resorption rate as shown by time-lapsed in vivo imaging of dynamic bone morphometry. Bone. doi:10.1016/j.bone.2011.08.035
Reginster JY, Sarlet N, Lecart MP (2005) Fractures in osteoporosis: the challenge for the new millennium. Osteoporos Int 16(Suppl 1):S1–S3
Bilezikian JP, Matsumoto T, Bellido T, Khosla S, Martin J, Recker RR, Heaney R, Seeman E, Papapoulos S, Goldring SR (2009) Targeting bone remodeling for the treatment of osteoporosis: summary of the proceedings of an ASBMR workshop. J Bone Miner Res 24:373–385
Egermann M, Goldhahn J, Schneider E (2005) Animal models for fracture treatment in osteoporosis. Osteoporos Int 16(Suppl 2):S129–S138
Mosekilde L, Danielsen CC, Knudsen UB (1993) The effect of aging and ovariectomy on the vertebral bone mass and biomechanical properties of mature rats. Bone 14:1–6
Fonseca D, Ward WE (2004) Daidzein together with high calcium preserve bone mass and biomechanical strength at multiple sites in ovariectomized mice. Bone 35:489–497
Brouwers JEM, Lambers FM, van Rietbergen B, Ito K, Huiskes R (2009) Comparison of bone loss induced by ovariectomy and neurectomy in rats analyzed by in vivo micro-CT. J Orthop Res 27:1521–1527
Silva MJ, Brodt MD, Uthgenannt BA (2004) Morphological and mechanical properties of caudal vertebrae in the SAMP6 mouse model of senile osteoporosis. Bone 35:425–431
Schulte FA, Lambers FM, Kuhn G, Müller R (2011) In vivo micro-computed tomography allows direct three-dimensional quantification of both bone formation and bone resorption parameters using time-lapsed imaging. Bone 48:433–442
Buie HR, Moore CP, Boyd SK (2008) Postpubertal architectural developmental patterns differ between the L3 vertebra and proximal tibia in three inbred strains of mice. J Bone Miner Res 23:2048–2059
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486
Thevenaz P, Ruttimann UE, Unser M (1998) A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 7:27–41
Webster DJ, Morley PL, van Lenthe GH, Müller R (2008) A novel in vivo mouse model for mechanically stimulated bone adaptation—a combined experimental and computational validation study. Comput Methods Biomech Biomed Eng 11:435–441
Christen P, van Rietbergen B, Lambers FM, Müller R, Ito K (2011) Bone morphology allows estimation of loading history in a murine model of bone adaptation. Biomech Model Mechanobiol. doi:10.1007/s10237-011-0327-x
Melton LJ 3rd, Christen D, Riggs BL, Achenbach SJ, Müller R, van Lenthe GH, Amin S, Atkinson EJ, Khosla S (2010) Assessing forearm fracture risk in postmenopausal women. Osteoporos Int 21:1161–1169
Bain SD, Bailey MC, Celino DL, Lantry MM, Edwards MW (1993) High-dose estrogen inhibits bone resorption and stimulates bone formation in the ovariectomized mouse. J Bone Miner Res 8:435–442
Kimble RB, Bain S, Pacifici R (1997) The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J Bone Miner Res 12:935–941
Parfitt AM (1994) Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem 55:273–286
Vande Berg BC, Malghem J, Lecouvet FE, Maldague B (2001) Normal bone marrow: dynamic aspects in magnetic resonance imaging. J Radiol 82:127–135
Donnelly EH, Nemhauser JB, Smith JM, Kazzi ZN, Farfan EB, Chang AS, Naeem SF (2010) Acute radiation syndrome: assessment and management. South Med J 103:541–546
Willinghamm MD, Brodt MD, Lee KL, Stephens AL, Ye J, Silva MJ (2010) Age-related changes in bone structure and strength in female and male BALB/c mice. Calcif Tissue Int 86:470–483
Glatt V, Canalis E, Stadmeyer L, Bouxsein ML (2007) Age-related changes in trabecular architecture differ in female and male C57Bl/6 J mice. J Bone Miner Res 22:1197–1207
Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S (2002) Changes in bone structure and mass with advancing age in the male C57Bl/6 J mouse. J Bone Miner Res 17:1044–1050
Brodt MD, Silva MJ (2010) Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res 25:2006–2015
Okada Y, Morimoto I, Ura K, Nakano Y, Tanaka Y, Nishida S, Nakamura T, Eto S (1998) Short-term treatment of recombinant murine interleukin-4 rapidly inhibits bone formation in normal and ovariectomized mice. Bone 22:361–365
Ward WE, Fonseca D (2007) Soy isoflavones and fatty acids: effects on bone tissue postovariectomy in mice. Mol Nutr Food Res 51:824–831
Waarsing JH, Day JS, Verhaar JA, Ederveen AG, Weinans H (2006) Bone loss dynamics result in trabecular alignment in aging and ovariectomized rats. J Orthop Res 24:926–935
Brouwers JEM, van Rietbergen B, Huiskes R (2007) No effects of in vivo micro-CT radiation on structural parameters and bone marrow cells in proximal tibia of Wistar rats detected after eight weekly scans. J Orthop Res 25:1325–1332
Acknowledgements
The authors gratefully acknowledge funding from the European Union for the Osteoporotic Virtual Physiological Human Project (FP7-ICT2008-223865), funding from the Whitaker Foundation, and computational time from the Swiss National Supercomputing Center (CSCS, Manno, Switzerland). We thank Peter Schwilch and Marco Hitz for technical assistance with the mouse holder and the statistical consulting team offered by the Seminar for Statistics at the ETH Zurich.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lambers, F.M., Kuhn, G., Schulte, F.A. et al. Longitudinal Assessment of In Vivo Bone Dynamics in a Mouse Tail Model of Postmenopausal Osteoporosis. Calcif Tissue Int 90, 108–119 (2012). https://doi.org/10.1007/s00223-011-9553-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-011-9553-6