Abstract
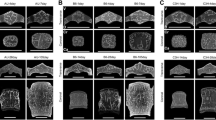
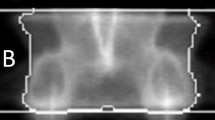
Most in vivo studies addressing the skeletal responses of mice to mechanical loading have targeted cortical bone. To investigate trabecular bone responses also we have developed a caudal vertebral axial compression device (CVAD) that transmits mechanical loads to compress the fifth caudal vertebra via stainless steel pins inserted into the forth and sixth caudal vertebral bodies. Here, we used the CVAD in C57BL/6 (B6) and C3H/Hej (C3H) female mice (15 weeks of age) to investigate whether the effect of regular bouts of mechanical stimulation on bone modeling and bone mass was dependent on dose and genotype. A combined micro-computed tomographic and dynamic histomorphometric analysis was carried out at the end of a 4-week loading regimen (3,000 cycles, 10 Hz, 3 x week) for load amplitudes of 0N, 2N, 4N and 8N. Significant increases in trabecular bone mass of 9 and 21% for loads of 4N and 8N, respectively, were observed in B6 mice. A significant increase of 10% in trabecular bone mass occurred for a load of 8N in the C3H strain. For other loads, no significant increases were detected. Both mouse strains exhibited substantial increases in trabecular bone formation rates for all loads, B6: 111% (2N), 86% (4N), 164% (8N), C3H: 41% (2N), 38% (4N), 141% (8N). Significant decreases in osteoclast number of 146 and 93% for a load of 8N were detected in B6 and C3H mice, respectively. These findings demonstrate that the effect of loading on the structural and functional parameters of bone is dose and genotype dependent. The caudal vertebral loading model established here is proposed for further studies addressing the molecular processes involved in the skeletal responses to mechanical stimuli.
Similar content being viewed by others
References
Akhter MP, Cullen DM et al (1998) Bone response to in vivo mechanical loading in two breeds of mice. Calcif Tissue Int 63(5): 442–449
Albright J (1987) The scientific basis of orthopaedics, 2nd edn. Appleton-Century Crofts, New York
Bajayo A, Goshen I et al (2005) Central IL-1 receptor signaling regulates bone growth and mass. Proc Natl Acad Sci USA 102(36): 12956–12961
Beamer WG, Shultz KL et al (2001) Quantitative trait loci for femoral and lumbar vertebral bone mineral density in C57BL/6J and C3H/HeJ inbred strains of mice. J Bone Miner Res 16(7): 1195–1206
Biewener AA, Fazzalari NL et al (1996) Adaptive changes in trabecular architecture in relation to functional strain patterns and disuse. Bone 19(1): 1–8
Boyd SK, Davison P et al (2006) Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone 39(4): 854–862
Buie HR, Moore CP et al (2008) Postpubertal architectural developmental patterns differ between the L3 vertebra and proximal tibia in three inbred strains of mice. J Bone Miner Res 23(12): 2048–2059
Chambers TJ, Evans M et al (1993) Induction of bone formation in rat tail vertebrae by mechanical loading. Bone Miner 20(2): 167–178
De Souza RL, Matsuura M et al (2005) Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 37(6): 810–818
Duncan RL, Turner CH (1995) Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 57(5): 344–358
Evans WJ (1998) Exercise and nutritional needs of elderly people: effects on muscle and bone. Gerodontology 15(1): 15–24
Fritton JC, Myers ER et al (2005) Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone 36(6): 1030–1038
Frost HM (1987) The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner 2(2): 73–85
Garman R, Gaudette G et al (2007) Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res 25(6): 732–740
Guo XE, Eichler MJ et al (2002) Quantification of a rat tail vertebra model for trabecular bone adaptation studies. J Biomech 35(3): 363–368
Hilderbrand T (1999) Direct three-demensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest and calcaneus. J Bone Miner Res 14: 1167–1174
Kesavan C, Mohan S et al (2005) Mechanical loading-induced gene expression and BMD changes are different in two inbred mouse strains. J Appl Physiol 99(5): 1951–1957
Kesavan C, Mohan S et al (2006) Identification of genetic loci that regulate bone adaptive response to mechanical loading in C57BL/6J and C3H/HeJ mice intercross. Bone 39((3): 634–643
Kodama Y, Umemura Y et al (2000) Exercise and mechanical loading increase periosteal bone formation and whole bone strength in C57BL/6J mice but not in C3H/Hej mice. Calcif Tissue Int 66(4): 298–306
Kohler T, Stauber M et al (2007) Automated compartmental analysis for high-throughput skeletal phenotyping in femora of genetic mouse models. Bone 41(4): 659–667
Koller DL, Schriefer J et al (2003) Genetic effects for femoral biomechanics, structure, and density in C57BL/6J and C3H/HeJ inbred mouse strains. J Bone Miner Res 18(10): 1758–1765
Kuruvilla SJ, Fox SD et al (2008) Site specific bone adaptation response to mechanical loading. J Musculoskelet Neuronal Interact 8(1): 71–78
Lambers FM, Kuhn G et al (2009) In vivo micro computed tomography allows monitoring of load induced microstructural bone adaptation. Bone 44(S2) (in press)
Lau KH, Kapur S et al (2006) Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J Biol Chem 281(14): 9576–9588
Marcus R (1995) Relationship of age-related decreases in muscle mass and strength to skeletal status. J Gerontol A Biol Sci Med Sci 50 Spec No: 86–87
Noh T, Gabet Y et al (2009) Lef1 haploinsufficient mice display a low turnover and low bone mass phenotype in a gender- and age-specific manner. PLoS One 4(5): e5438
Ofek O, Karsak M et al (2006) Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA 103(3): 696–701
Ozcivici E, Garman R et al (2007) High-frequency oscillatory motions enhance the simulated mechanical properties of non-weight bearing trabecular bone. J Biomech 40(15): 3404–3411
Parfitt AM (1988) Bone histomorphometry: proposed system for standardization of nomenclature, symbols, and units. Calcif Tissue Int 42(5): 284–286
Robling AG, Turner CH (2002) Mechanotransduction in bone: genetic effects on mechanosensitivity in mice. Bone 31(5): 562–569
Rubin CT, Lanyon LE (1985) Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int 37(4): 411–417
Rubin CT, Sommerfeldt DW et al (2001) Inhibition of osteopenia by low magnitude, high-frequency mechanical stimuli. Drug Discov Today 6(16): 848–858
Turner CH, Hsieh YF et al (2000) Genetic regulation of cortical and trabecular bone strength and microstructure in inbred strains of mice. J Bone Miner Res 15(6): 1126–1131
Webster DJ, Morley PL et al (2008) A novel in vivo mouse model for mechanically stimulated bone adaptation—a combined experimental and computational validation study. Comput Methods Biomech Biomed Engin 11(5): 435–441
Xing W, Baylink D et al (2005) Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice. J Cell Biochem 96(5): 1049–1060
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Webster, D., Wasserman, E., Ehrbar, M. et al. Mechanical loading of mouse caudal vertebrae increases trabecular and cortical bone mass-dependence on dose and genotype. Biomech Model Mechanobiol 9, 737–747 (2010). https://doi.org/10.1007/s10237-010-0210-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-010-0210-1