Abstract
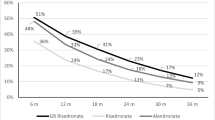
Upper gastrointestinal (GI) side effects are a known complication of therapy with oral aminobisphosphonates, but it is currently unclear if bisphosphonate type or formulation influences the risk of developing side effects. Here, we performed a retrospective cohort study to determine if patients who switched from weekly risedronate to weekly alendronate had an increased risk of upper GI side events. The study utilized The Health Improvement Network (THIN) database, which contained anonymous medical records from 390 general practices in the United Kingdom. The study was performed following the introduction of generic alendronate preparations, by which point 94% of alendronate prescriptions were for the generic formulation. We identified 3,446 patients who had been stabilized on risedronate 35 mg/week, of whom 530 were switched to alendronate 70 mg/week. The risk of developing a GI adverse event was higher in patients who switched to alendronate compared with those who remained on risedronate (hazard ratio [HR] = 1.85, 95% confidence interval [CI] 1.26–2.72). The risk was even greater in the subgroup of patients with a history of upper GI events (HR = 3.18, 95% CI 2.79–3.63) but was also observed in patients with no history of GI events (HR = 1.76, 95% CI 1.15–2.69). We conclude that switching patients who are stabilized on risedronate to alendronate is associated with an increased risk of GI adverse effects. This could lead to reduced compliance and reduced therapeutic effectiveness, which might offset the cost savings of using the generic formulation.
Similar content being viewed by others
References
Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster JY, Borgstrom F, Rizzoli R (2008) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 19:399–428
Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR (2000) Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. Clin Endocrinol Metab 85:4118–4124
Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B (1999) Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporosis Int 9:461–468
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH III, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group [see comments]. JAMA 282:1344–1352
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporosis Int 11:83–91
McClung MR, Guesens P, Miller PD, Zippel H, Roux C, Roux C, Adami S, Fogelman I, Diamond T, Meunier PJ, Wasnich RD, Greenwald M, Kaufman JM, Chestnut CH III, Reginster JY (2001) Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 344:333–340
Dansereau RJ, Crail DJ, Perkins AC (2008) In vitro disintegration and dissolution studies of once-weekly copies of alendronate sodium tablets (70 mg) and in vivo implications. Curr Med Res Opin 24:1137–1145
Lanza FL (2002) Gastrointestinal adverse effects of bisphosphonates: etiology, incidence and prevention. Treat Endocrinol 1:37–43
de Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, Pryor-Tillotson S, Seleznick MJ, Pinkas H, Wang KK (1996) Esophagitis associated with the use of alendronate. N Engl J Med 335:1016–1021
Kikendall JW, Friedman AC, Oyewole MA, Fleischer D, Johnson LF (1983) Pill-induced esophageal injury. Case reports and review of the medical literature. Dig Dis Sci 28:174–182
Bonnick S, Saag KG, Kiel DP, McClung M, Hochberg M, Burnett SA, Sebba A, Kagan R, Chen E, Thompson DE, de Papp AE (2006) Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab 91:2631–2635
Epstein S, Cryer B, Ragi S, Zanchetta JR, Walliser J, Chow J, Johnson MA, Leyes AE (2003) Disintegration/dissolution profiles of copies of Fosamax (alendronate). Curr Med Res Opin 19:781–789
Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C (2004) The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int 15:1003–1008
Sheehy O, Kindundu CM, Barbeau M, LeLorier J (2009) Differences in persistence among different weekly oral bisphosphonate medications. Osteoporos Int 20:1369–1376
Halkin H, Dushenat M, Silverman B, Shalev V, Loebstein R, Lomnicky Y, Friedman N (2007) Brand versus generic alendronate: gastrointestinal effects measured by resource utilization. Ann Pharmacother 41:29–34
Himmel W, Simmenroth-Nayda A, Niebling W, Ledig T, Jansen RD, Kochen MM, Gleiter CH, Hummers-Pradier E (2005) What do primary care patients think about generic drugs? Int J Clin Pharmacol Ther 43:472–479
Kjoenniksen I, Lindbaek M, Granas AG (2006) Patients’ attitudes towards and experiences of generic drug substitution in Norway. Pharm World Sci 28:284–289
Cramer JA, Gold DT, Silverman SL, Lewiecki EM (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 18:1023–1031
Huybrechts KF, Ishak KJ, Caro JJ (2006) Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone 38:922–928
Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81:1013–1022
Weycker D, Macarios D, Edelsberg J, Oster G (2007) Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int 18:271–277
Penning-van Beest FJ, Goettsch WG, Erkens JA, Herings RM (2006) Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther 28:236–242
Rossini M, Bianchi G, Di MO, Giannini S, Minisola S, Sinigaglia L, Adami S (2006) Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int 17:914–921
Acknowledgments
This research was funded by The Alliance for Better Bone Health (Procter & Gamble Pharmaceuticals and Sanofi-Sventis U.S.). The authors received editorial/writing support in the preparation of this manuscript funded by The Alliance for Better Bone Health; the authors, however, are fully responsible for all content and editorial decisions and received no financial support or other form of compensation related to the development of the report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Professor Ralston acted as a consultant for Proctor & Gamble Pharmaceuticals at the time this study was performed. Professor Ralston currently acts as a consultant for Warner Chilcott, Novartis, and Merck and is in receipt of research grant support from Wyeth and Novartis. Dr Kou, Wick-Urban and Steinbuch were employees of Procter & Gamble Pharmaceuticals at the time this study was performed.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00223-010-9430-8
Rights and permissions
About this article
Cite this article
Ralston, S.H., Kou, T.D., Wick-Urban, B. et al. Risk of Upper Gastrointestinal Tract Events in Risedronate Users Switched to Alendronate. Calcif Tissue Int 87, 298–304 (2010). https://doi.org/10.1007/s00223-010-9401-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-010-9401-0