Abstract
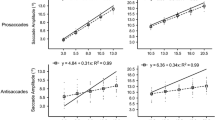
Saccade endpoints are most frequently characterized by an undershooting bias. Notably, however, some evidence suggests that saccades can be made to systematically under- or overshoot a target based on the magnitude of the eccentricities within a given block of trials (i.e., the oculomotor range effect hypothesis). To address that issue, participants completed stimulus-driven saccades in separate blocks of trials (i.e., proximal vs. distal) that entailed an equal number of targets but differed with respect to the magnitude of their eccentricities. In the proximal block, target eccentricities were 3.0°, 5.5°, 8.0°, 10.5° and 13.0°, whereas in the distal block target eccentricities were 10.5°, 13.0°, 15.5°, 18.0° and 20.5°. If the range effect represents a tenable hypothesis, then the magnitude of target eccentricities within each block should selectively influence saccade endpoint bias. More specifically, the eccentricities common to the proximal and distal blocks (i.e., 10.5° and 13.0°) should elicit a systematic under- and overshooting bias, respectively. Results for the proximal and distal blocks showed a reliable undershooting bias across target eccentricities, and a direct comparison of the common eccentricities indicated that the undershooting bias was not modulated between blocks. Moreover, our results show that the presence of online target vision did not influence the undershooting bias. Thus, the present findings provide no support for an oculomotor range effect; rather, results evince the mediation of saccades via a control strategy that minimizes movement time and/or the energy requirements of the response.




Similar content being viewed by others
Notes
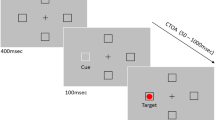
We define the saccades studied here as being stimulus-driven given that the onset of an exogenously presented visual target served as the response imperative. Notably, stimulus-driven saccades involve direct spatial mapping between stimulus and response, and their exogenous presentation entails minimal top-down influence (Rossetti et al. 2005).
Saccade amplitudes in the left and right visual fields did not reliably differ (F < 1). For that reason, visual field was a collapsed factor in our ANOVA model.
Kapoula (1985) interpreted her data via descriptive statistics (i.e., means) and did not include inferential analyses. However, our inferential analyses of the participant-specific data associated with Kapoula’s proximal block (see Table 1 of that work) via single-sample t statistics contrasting saccade amplitudes to veridical target location yielded the following 2.7°: t(3) = 22.26, p < 0.001; 4.4°: t(3) = 0.65, p = 0.56; 6.1°: t(3) = −0.78, p = 0.48; 7.8°: t(3) = −2.86, p = 0.06; 9.5°: t(3) = −3.35, p < 0.05. Thus, only the most proximal and most distal target eccentricities produced amplitudes that reliably differed from veridical target location. Kapoula’s distal block included two participants; we therefore did not submit those data to inferential examination.
References
Abrams RA, Meyer DE, Kornblum SK (1989) Speed and accuracy of saccadic eye movements: characteristics of impulse variability in the oculomotor system. J Exp Psychol 15:529–543
Becker W (1989) Metrics. In: Wurtz RH, Goldberg ME (eds) The neurobiology of saccadic eye movements. Elsevier, Amsterdam, pp 13–67
Becker W, Fuchs AF (1969) Further properties of the human saccadic system: eye movements and correction saccades with and without visual fixation points. Visi Res 9:1247–1258
Binsted G, Elliott D (1999) Ocular perturbations and retinal/extraretinal information: the coordination of saccadic and manual movements. Exp Brain Res 127:193–206
Brainard DH (1997) The psychophysics toolbox. Spat Vis 10:433–436
Dafoe JM, Armstrong IT, Munoz DP (2007) The influence of stimulus direction and eccentricity on pro- and anti-saccades in humans. Exp Brain Res 179:563–570
Deubel H, Wolf W, Hauske G (1986) Adaptive gain control of saccadic eye movements. Hum Neurobiol 5:245–253
Ehresman C, Saucier D, Heath M, Binsted G (2008) Online corrections can produce illusory bias during closed-loop pointing. Exp Brain Res 188:371–378
Elliott D, Hansen S, Mendoza J, Tremblay L (2004) Learning to optimize speed, accuracy and energy expenditure: a framework for understanding speed-accuracy relations in goal-directed aiming. J Mot Behav 36:339–351
Engelbrecht SE, Berthier NE, O’Sullivan LP (2003) The undershoot bias: learning to act optimally under uncertainty. Psychol Sci 14:257–261
Evdokimidis I, Tsekou H, Smyrnis N (2006) The mirror antisaccade task: direction-amplitude interaction and spatial accuracy characteristics. Exp Brain Res 174:304–311
Fitts PM, Peterson JR (1964) Information capacity of discrete motor responses. J Exp Psychol 67:103–112
Gaveau V, Martin O, Prablanc C, Pélisson D, Urquizar C, Desmurget M (2003) On-line modification of saccadic eye movements by retinal signals. NeuroReport 14:875–878
Gerardin P, Gaveau V, Pèlisson D, Prablanc C (2011) Integration of visual information for saccade production. Hum Mov Sci 30:1009–1021
Goodale MA (2011) Transforming vision into action. Vis Res 51:1567–1587
Hallett PE (1978) Primary and secondary saccades to goals defined by instructions. Vis Res 18:1279–1296
Harris CM (1995) Does saccadic undershoot minimize saccadic flight-time? A Monte-Carlo study. Vis Res 35:691–701
Harris CM, Jacobs M, Shawkat F, Taylor D (1993) The development of saccadic accuracy in the first seven months. Clin Vision Sci 8:85–96
Heath M, Dunham K, Binsted G, Godbolt B (2010) Antisaccades exhibit diminished online control relative to prosaccades. Exp Brain Res 203:743–752
Heath M, Weiler J, Marriott K, Welsh TN (2011) Vector inversion diminishes the online control of antisaccades. Exp Brain Res 209:117–127
Kapoula Z (1985) Evidence for a range effect in the saccadic system. Vis Res 25:1155–1157
Kapoula Z, Robinson DA (1986) Saccadic undershoot is not inevitable: saccades can be accurate. Vis Res 26:735–743
Khan MA, Elliot D, Coull J, Chua R, Lyons J (2002) Optimal control strategies under different feedback schedules: kinematic evidence. J Mot Behav 34:45–57
Knox PC, Bruno N (2007) When does action resist visual illusion? The effect of Müller–Lyer stimuli on reflexive and voluntary saccades. Exp Brain Res 181:277–287
Kowler E, Blaser E (1995) The accuracy and precision of saccades to small and large targets. Vis Res 35:1741–1754
Krappmann P, Everling S, Flohr H (1998) Accuracy of visually and memory-guided antisaccades in man. Vis Res 38:2979–2985
Oliveira FTP, Elliott D, Goodman D (2005) Energy-minimization bias: compensating for intrinsic influence of energy-minimization mechanisms. Mot Control 9:101–114
Prablanc C, Jeannerod M (1975) Corrective saccades: dependence on retinal reafferent signals. Vis Res 15:465–469
Robinson DA (1973) Models of the saccadic eye movement control system. Kybernetik 14:71–83
Rossetti Y, Revol P, McIntosh R, Pisella L, Rode G, Danckert J, Tilikete C, Dijkerman HC, Boisson D, Vighetto A, Michel F, Milner AD (2005) Visually guided reaching: bilateral posterior parietal lesions cause a switch from fast visuomotor to slow cognitive control. Neuropsychologia 43:162–177
Snedecor GW, Cochran WG (1980) Statistical methods. Iowa State University Press, Ames, IA
Todorov E, Jordan MI (2002) Optimal feedback control as a theory of motor coordination. Nat Neurosci 5:1226–1235
Vitu F (1991) Against the existence of a range effect during reading. Vis Res 31:2009–2015
Weber RB, Daroff RB (1971) The metrics of horizontal saccadic eye movements in normal humans. Vis Res 11:921–928
Weiler J, Heath M (2012a) Task-switching in oculomotor control: unidirectional switch-cost when alternating between pro- and antisaccades. Neurosci Lett 530:150–154
Weiler J, Heath M (2012b) The prior-antisaccade effect influences the planning and online control of prosaccades. Exp Brain Res 216:545–552
Weiler J, Holmes SA, Mulla A, Heath M (2011) Pro- and antisaccades: dissociating stimulus and response influences the online control of saccade trajectories. J Mot Behav 43:375–381
West GL, Welsh TN, Pratt J (2009) Saccadic trajectories receive online correction: evidence for a feedback-based system of oculomotor control. J Mot Behav 41:117–127
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada, and by an Academic Development Fund and Faculty Scholar Award from the University of Western Ontario.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gillen, C., Weiler, J. & Heath, M. Stimulus-driven saccades are characterized by an invariant undershooting bias: no evidence for a range effect. Exp Brain Res 230, 165–174 (2013). https://doi.org/10.1007/s00221-013-3640-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-013-3640-z