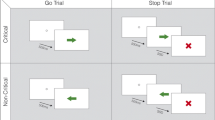
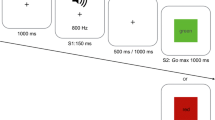
Abstract
It has been proposed that the subthalamic nucleus (STN) mediates response inhibition and conflict resolution through the fronto-basal ganglia pathways. Our aim was to compare the effects of deep brain stimulation (DBS) of the STN on reactive and proactive inhibition and conflict resolution in Parkinson’s disease using a single task. We used the conditional Stop signal reaction time task that provides the Stop signal reaction time (SSRT) as a measure of reactive inhibition, the response delay effect (RDE) as a measure of proactive inhibition and conflict-induced slowing (CIS) as a measure of conflict resolution. DBS of the STN significantly prolonged SSRT relative to stimulation off. However, while the RDE measure of proactive inhibition was not significantly altered by DBS of the STN, relative to healthy controls, RDE was significantly lower with DBS off but not DBS on. DBS of the STN did not alter the mean CIS but produced a significant differential effect on the slowest and fastest RTs on conflict trials, further prolonging the slowest RTs on the conflict trials relative to DBS off and to controls. These results are the first demonstration, using a single task in the same patient sample, that DBS of the STN produces differential effects on reactive and proactive inhibition and on conflict resolution, suggesting that these effects are likely to be mediated through the impact of STN stimulation on different fronto-basal ganglia pathways: hyperdirect, direct and indirect.






Similar content being viewed by others
References
Aron AR (2011) From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry 69(12):e55–e68. doi:10.1016/j.biopsych.2010.07.024
Aron AR, Poldrack RA (2006) Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci 26(9):2424–2433
Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA (2007) Triangulating a cognitive control network using diffusion-weighted magnetic Resonance imaging (MRI) and functional MRI. J Neurosci 27(14):3743–3752
Ballanger B, van Eimeren T, Moro E, Lozano AM, Hamani C, Boulinguez P, Pellecchia G, Houle S, Poon YY, Lang AE, Strafella AP (2009) Stimulation of the subthalamic nucleus and impulsivity: release your horses. Ann Neurol 66(6):817–824. doi:10.1002/ana.21795
Baunez C, Nieoullon A, Amalric M (1995) In a rat model of Parkinsonism, lesions of the subthalamic nucleus reverse increases of reaction time but induce a dramatic premature responding deficit. J Neurosci 15(10):6531–6541
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571
Brown RG, Dowsey PL, Brown P, Jahanshahi M, Pollak P, Benabid AL, Rodriguez-Oroz MC, Obeso J, Rothwell JC (1999) Impact of deep brain stimulation on upper limb akinesia in Parkinson’s disease. Ann Neurol 45(4):473–488
Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ (2011) Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci. doi:10.1038/nn.2925
Chen X, Scangos KW, Stuphorn V (2010) Supplementary motor area exerts proactive and reactive control of arm movements. J Neurosci 30(44):14657–14675. doi:10.1523/JNEUROSCI.2669-10.2010
Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S (2009) Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci 29(50):15870–15877. doi:10.1523/JNEUROSCI.3645-09.2009
De Jong R, Coles MG, Logan GD (1995) Strategies and mechanisms in nonselective and selective inhibitory motor control. J Exp Psychol Hum Percept Perform 21(3):498–511
Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW (2008) Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex 18(1):178–188
Fahn S, Elton RL, Members of the UPDRS Development Committee (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Clane DB (eds) Recent developments in Parkinson’s disease. Macmillan Healthcare Information, Florham Park, pp 153–163
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Frank MJ (2006) Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw 19(8):1120–1136
Frank MJ, Samanta J, Moustafa AA, Sherman SJ (2007) Hold your horses: impulsivity, deep brain stimulation, and medication in Parkinsonism. Science 318(5854):1309–1312. doi:10.1126/science.1146157
Hershey T, Revilla FJ, Wernle A, Gibson PS, Dowling JL, Perlmutter JS (2004) Stimulation of STN impairs aspects of cognitive control in PD. Neurology 62(7):1110–1114
Hershey T, Campbell MC, Videen TO, Lugar HM, Weaver PM, Hartlein J, Karimi M, Tabbal SD, Perlmutter JS (2010) Mapping Go-No-Go performance within the subthalamic nucleus region. Brain. doi:10.1093/brain/awq256
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, Lang AE, Lozano AM (1998) Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson’s disease. Ann Neurol 44(4):622–628. doi:10.1002/ana.410440407
Jahanshahi M, Ardouin CM, Brown RG, Rothwell JC, Obeso J, Albanese A, Rodriguez-Oroz MC, Moro E, Benabid AL, Pollak P, Limousin-Dowsey P (2000) The impact of deep brain stimulation on executive function in Parkinson’s disease. Brain 123(Pt 6):1142–1154
Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR (2010) Responding with restraint: what are the neurocognitive mechanisms? J Cogn Neurosci 22(7):1479–1492. doi:10.1162/jocn.2009.21307
Jahfari S, Waldorp L, van den Wildenberg WP, Scholte HS, Ridderinkhof KR, Forstmann BU (2011) Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J Neurosci 31(18):6891–6899. doi:10.1523/JNEUROSCI.5253-10.2011
Kuhn AA, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, Schneider GH, Brown P (2009) Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol 215(2):380–387. doi:10.1016/j.expneurol.2008.11.008
Li CS, Huang C, Constable RT, Sinha R (2006) Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci 26(1):186–192. doi:10.1523/JNEUROSCI.3741-05.2006
Li CS, Yan P, Sinha R, Lee TW (2008) Subcortical processes of motor response inhibition during a stop signal task. Neuroimage 41(4):1352–1363. doi:10.1016/j.neuroimage.2008.04.023
Lim SY, O’Sullivan SS, Kotschet K, Gallagher DA, Lacey C, Lawrence AD, Lees AJ, O’Sullivan DJ, Peppard RF, Rodrigues JP, Schrag A, Silberstein P, Tisch S, Evans AH (2009) Dopamine dysregulation syndrome, impulse control disorders and punding after deep brain stimulation surgery for Parkinson’s disease. J Clin Neurosci 16(9):1148–1152. doi:10.1016/j.jocn.2008.12.010
Logan GD, Cowan WB (1984) On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev 91(3):295–327
Mirabella G, Iaconelli S, Romanelli P, Modugno N, Lena F, Manfredi M, Cantore G (2011) Deep brain stimulation of subthalamic nuclei affects arm response inhibition in Parkinson’s patients. Cereb Cortex. doi:10.1093/cercor/bhr187
Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF (2010) Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci USA 107(30):13240–13245. doi:10.1073/pnas.1000674107
Obeso JA, Guridi J, De Long M (1997) Surgery for Parkinson’s disease. J Neurol Neurosurg Psychiatry 62:2–8
Obeso I, Wilkinson L, Casabona E, Bringas ML, Alvarez M, Alvarez L, Pavon N, Rodriguez-Oroz MC, Macias R, Obeso JA, Jahanshahi M (2011a) Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson’s disease. Exp Brain Res 212(3):371–384. doi:10.1007/s00221-011-2736-6
Obeso I, Wilkinson L, Jahanshahi M (2011b) Levodopa medication does not influence motor inhibition or conflict resolution in a conditional stop-signal task in Parkinson’s disease. Exp Brain Res 213(4):435–445. doi:10.1007/s00221-011-2793-x
Ray NJ, Jenkinson N, Brittain J, Holland P, Joint C, Nandi D, Bain PG, Yousif N, Green A, Stein JS, Aziz TZ (2009) The role of the subthalamic nucleus in response inhibition: evidence from deep brain stimulation for Parkinson’s disease. Neuropsychologia 47(13):2828–2834. doi:10.1016/j.neuropsychologia.2009.06.011
Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto J-L, Pollak P, Rehncrona S, Kulisevsky J, Albanese A, Volkmann J, Hariz MI, Quinn NP, Speelman JD, Guridi J, Zamarbide I, Gironell A, Molet J, Pascual-Sedano B, Pidoux B, Bonnet AM, Agid Y, Xie J, Benabid AL, Lozano AM, Saint-Cyr J, Romito L, Contarino MF, Scerrati M, Fraix V, Van Blercom N (2005) Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain 128:2240–2249
Rubia K, Smith AB, Brammer MJ, Taylor E (2003) Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage 20(1):351–358
Swann N, Poizner H, Houser M, Gould S, Greenhouse I, Cai W, Strunk J, George J, Aron AR (2011) Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the Beta frequency band: a scalp EEG study in Parkinson’s disease. J Neurosci 31(15):5721–5729. doi:10.1523/JNEUROSCI.6135-10.2011
Thobois S, Hotton GR, Pinto S, Wilkinson L, Limousin-Dowsey P, Brooks DJ, Jahanshahi M (2007) STN stimulation alters pallidal-frontal coupling during response selection under competition. J Cereb Blood Flow Metab 27(6):1173–1184. doi:10.1038/sj.jcbfm.9600425
van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH (2006) Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J Cogn Neurosci 18(4):626–636
Verbruggen F, Logan GD (2009a) Automaticity of cognitive control: goal priming in response-inhibition paradigms. J Exp Psychol Learn Mem Cogn 35(5):1381–1388. doi:10.1037/a0016645
Verbruggen F, Logan GD (2009b) Proactive adjustments of response strategies in the stop-signal paradigm. J Exp Psychol Hum Percept Perform 35(3):835–854. doi:10.1037/a0012726
Verbruggen F, Aron AR, Stevens MA, Chambers CD (2010) Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc Natl Acad Sci USA 107(31):13966–13971. doi:10.1073/pnas.1001957107
Verbruggen F, Chambers CD, Logan GD (2013) Fictitious inhibitory differences: how skewness and slowing distort the estimation of stopping latencies. Psychol Sci [Epub ahead of print]
Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF (2005) Function of striatum beyond inhibition and execution of motor responses. Hum Brain Mapp 25(3):336–344. doi:10.1002/hbm.20111
Voon V, Kubu C, Krack P, Houeto JL, Troster AI (2006) Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord 21(Suppl 14):S305–S327. doi:10.1002/mds.20963
Wichmann T, Bergman H, DeLong MR (1994) The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol 72(2):521–530
Wiener M, Magaro CM, Matell MS (2008) Accurate timing but increased impulsivity following excitotoxic lesions of the subthalamic nucleus. Neurosci Lett 440(2):176–180. doi:10.1016/j.neulet.2008.05.071
Witt K, Pulkowski U, Herzog J, Lorenz D, Hamel W, Deuschl G, Krack P (2004) Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease. Arch Neurol 61(5):697–700. doi:10.1001/archneur.61.5.697
Wylie SA, Ridderinkhof KR, Elias WJ, Frysinger RC, Bashore TR, Downs KE, van Wouwe NC, van den Wildenberg WP (2010) Subthalamic nucleus stimulation influences expression and suppression of impulsive behaviour in Parkinson’s disease. Brain 133(Pt 12):3611–3624. doi:10.1093/brain/awq239
Yugeta A, Terao Y, Fukuda H, Hikosaka O, Yokochi F, Okiyama R, Taniguchi M, Takahashi H, Hamada I, Hanajima R, Ugawa Y (2010) Effects of STN stimulation on the initiation and inhibition of saccade in Parkinson disease. Neurology 74(9):743–748. doi:10.1212/WNL.0b013e3181d31e0b
Zandbelt BB, Vink M (2010) On the role of the striatum in response inhibition. PLoS ONE 5(11):e13848. doi:10.1371/journal.pone.0013848
Acknowledgments
We are grateful to all the participants. This research was supported by a PhD studentship from Fundación Caja Madrid, Spain (IO), and a Post-doctoral Career Development Fellowship from Parkinson’s UK (LW).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Obeso, I., Wilkinson, L., Rodríguez-Oroz, MC. et al. Bilateral stimulation of the subthalamic nucleus has differential effects on reactive and proactive inhibition and conflict-induced slowing in Parkinson’s disease. Exp Brain Res 226, 451–462 (2013). https://doi.org/10.1007/s00221-013-3457-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-013-3457-9