Abstract
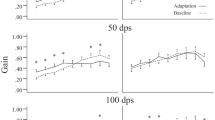
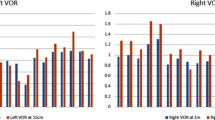
The angular vestibulo-ocular reflex (aVOR) has a fast pathway, which mediates compensatory eye movements, and a slow (velocity storage) pathway, which determines its low frequency characteristics and orients eye velocity toward gravity. We have proposed that motion sickness is generated through velocity storage, when its orientation vector, which lies close to the gravitational vertical, is misaligned with eye velocity during head motion. The duration of the misalignment, determined by the dominant time constant of velocity storage, causes the buildup of motion sickness. To test this hypothesis, we studied bilateral labyrinthine-defective subjects with short vestibular time constants but normal aVOR gains for their motion sickness susceptibility. Time constants and gains were taken from rotational responses. Motion sickness was generated by rolling the head while rotating, and susceptibility was assessed by the number of head movements made before reaching intolerable levels of nausea. More head movements signified lower motion sickness susceptibility. Labyrinthine-defective subjects made more head movements on their first exposure to roll while rotating than normals (39.8 ± 7.2 vs 13.7 ± 5.5; P < 0.0001). Normals were tested eight times, which habituated their time constants and reduced their motion sickness susceptibility. Combining data from all subjects, there was a strong inverse relationship between time constants and number of head movements (r = 0.94), but none between motion sickness susceptibility and aVOR gains. This provides further evidence that motion sickness is generated through velocity storage, not the direct pathway, and suggests that motion sickness susceptibility can be reduced by reducing the aVOR time constant.



Similar content being viewed by others
References
Balaban CD (1999) Vestibular autonomic regulation (including motion sickness and the mechanism of vomiting). Curr Opin Neurol 12:29–33
Baloh RW, Hess K, Honrubia V, Yee RD (1984) Low and high frequency sinusoidal rotational testing in patients with vestibular lesions. Acta Otolaryngol Suppl 406:189–193
Baloh RW, Jacobson K, Honrubia V (1989) Idiopathic bilateral vestibulopathy. Neurology 39:272–275
Bard P (1945) Subcommittee on motion sickness; Committee on aviation medicine. In: National Research Council, National Academy of Science
Black FO, Gianna-Poulin C, Pesznecker SC (2001) Recovery from vestibular ototoxicity. Otol Neurotol 22:662–671
Bles W (1988) Coriolis effects and motion sickness modeling. Brain Res Bull 543–549
Bos JE, Bles W, de Graaf B (2002) Eye movements to yaw, pitch, and roll about vertical and horizontal axes: adaptation and motion sickness. Aviat Space Environ Med 73:436–434
Bouyer LJ, Watt DG (1996) “Torso rotation” experiments; 1: adaptation to motion sickness does not correlate with changes in VOR gain. J Vestib Res 6:367–375
Brandt T (1999) Vertigo. Springer, London
Büttner U, Waespe W (1981) Vestibular nerve activity in the alert monkey during vestibular and optokinetic nystagmus. Exp Brain Res 41:310–315
Cheung BS, Howard IP, Money KE (1991) Visually induced sickness in normal and bilaterally labyrinthine-defective subjects. Aviat Space Environ Med 62:527–531
Clément G, Deguine O, Parant M, Costes-Salon MC, Vasseur-Clausen P, Pavy-LeTraon A (2001) Effects of cosmonaut vestibular training on vestibular function prior to spaceflight. Eur J Appl Physiol 85:539–545
Cohen B, Uemura T, Takemori S (1973) Effects of labyrinthectomy on optokinetic nystagmus (OKN) and optokinetic after-nystagmus (OKAN). Int J Equilb Res 3:88–93
Cohen B, Matsuo V, Raphan T (1977) Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol (Lond) 270:321–344
Cohen B, Helwig D, Raphan T (1987) Baclofen and velocity storage: a model of the effects of the drug on the vestibulo-ocular reflex in the rhesus monkey. J Physiol 393:703–725
Cohen H, Cohen B, Raphan T, Waespe W (1992) Habituation and adaptation of the vestibulo-ocular reflex: a model of differential control by the vestibulo-cerebellum. Exp Brain Res 90:526–538
Cohen B, Dai M, Raphan T (2003) The critical role of velocity storage in production of motion sickness. Ann NY Acad Sci 1004:359–376
Correia MJ, Perachio AA, Dickman JD, Kozlovskaya IB, Sirota MG, Yakushin SB, Beloozerova IN (1992) Post-flight changes in yaw responses of horizontal semicircular canal afferents in rhesus monkey following 14 days of microgravity. In: XVIIth Barany Society Meeting, Czechoslovakia, June 1–5, pp 188–189
Dai M, Raphan T, Cohen B (1991) Spatial orientation of the vestibular system: dependence of optokinetic after nystagmus on gravity. J Neurophysiol 66:1422–1438
Dai M, Klein A, Cohen B, Raphan T (1999) Model-based study of the human cupular time constant. J Vestib Res 9:293–301
Dai M, Kunin M, Raphan T, Cohen B (2003) The relation of motion sickness to the spatial-temporal properties of velocity storage. Exp Brain Res 151:173–189
Dai M, Raphan T, Cohen B (2005) Effects of baclofen on the angular vestibulo-ocular reflex. Exp Brain Res 1–10
Dichgans J, Schmidt CL, Graf W (1973) Visual input improves the speedometer function of the vestibular nuclei in the goldfish. Exp Brain Res 18:319–322
Dickman JD, Correia MJ (1989) Responses of pigeon horizontal semicircular canal afferent fibers. II. High-frequency mechanical stimulation. J Neurophysiol 62:1102–1112
Fanelli R, Raphan T, Schnabolk C (1990) Neural network modelling of eye compensation during off-vertical axis rotation. Neural Netw 3:265–276
Fernández C, Goldberg JM (1971) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol 34:661–675
Fetter M, Zee DS (1988) Recovery from unilateral labyrinthectomy in rhesus monkey. J Neurophysiol 59:370–393
Galiana HL, Smith HL, Katsarkas A (2001) Modelling non-linearities in the vestibulo-ocular reflex (VOR) after unilateral or bilateral loss of peripheral vestibular function. Exp Brain Res 137:369–386
Goldberg JM, Fernandez C (1971) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to angular accelerations. J Neurophysiol 34:635–660
Golding JF, Kerguelen M (1992) A comparison of the nauseogenic potential of low-frequency vertical versus horizontal linear oscillation. Aviat Space Environ Med 63:491–497
Gordon CR, Spitzer O, Doweck I, Shupak A, Gadoth N (1996) The vestibulo-ocular reflex and seasickness susceptibility. J Vestib Res 6:229–233
Graybiel A, Wood CD (1969) Rapid vestibular adaptation in a rotary environment by means of controlled head movements. Aerosp Med 40:638–643
Graybiel A, Miller EF II, Homick JL (1975) Individual differences in susceptibility to motion sickness among six Skylab astronauts. Acta Astronaut 2:155–174
Guedry FE, Benson AJ (1978) Coriolis cross-coupling effects: disorienting and nauseogenic or not? Aviat Space Environ Med 49:29–35
Guedry FE, Collins WE, Graybiel A (1964) Vestibular habituation during repetitive complex stimulation: a study of transfer effects. J Appl Physiol 19:1005–1015
Hain T, Buettner UE (1990) Static roll and the vestibulo-ocular reflex (VOR). Exp Brain Res 82:463–471
Hain TC, Zee DS, Maria BL (1988) Tilt suppression of vestibulo-ocular reflex in patients with cerebellar lesions. Acta Otolaryngol 105:13–20
Hecht H, Kavelaars J, Cheung CC, Young LR (2001) Orientation illusions and heart-rate changes during short-radius centrifugation. J Vestib Res 11:115–127
Hoffer ME, Gottshall K, Kopke RD, Weisskopf P, Moore R, Allen KA, Wester D (2003) Vestibular testing abnormalities in individuals with motion sickness. Otol Neurotol 24:633–636
Holstein GR, Martinelli GP, Cohen B (1992) L-balcofen-sensitive GABAb binding sites in the medial vestibular nucleus localized by immunocytochemistry. Brain Res 581:175–180
Holstein GR, Martinelli GP, Wearne S, Cohen B (1999) Ultrastructure of vestibular commissural neurons related to velocity storage in the monkey. Neuroscience 93:155–170
Igarashi M, Kobayashi K (1985) Space motion sickness and space vestibulology. J UOEH 7 Suppl:228–236
Johnson WH, Sunahara FA, Landolt JP (1999) Importance of the vestibular system in visually induced nausea and self-vection. J Vestib Res 9:83–87
Kucharczyk J, Stewart DJ, Miller AD (1991) Nausea And Vomitting, Recent Research And Clinical Advances. CRC Press, Boca Raton, Ann Arbor, Boston, London
Lackner JR, Graybiel A (1986) The effective intensity of Coriolis, cross-coupling stimulation is gravitoinertial force dependent: implications for space motion sickness. Aviat Space Environ Med 57:229–235
Lackner JR, Graybiel A (1994) Use of promethazine to hasten adaptation to provocative motion. J Clin Pharmacol 34:644–648
Lee MY, Kim MS, Park BR (2004) Adaptation of the horizontal vestibuloocular reflex in pilots. Laryngoscope 114:897–902
Lisberger SG, Miles FA, Optican LM, Eighmy BB (1981) Optokinetic response in monkey: underlying mechanisms and their sensitivity to long-term adaptive changes in vestibulo-ocular reflex. J Neurophysiol 45:869–890
Lorente de No R (1933) Vestibular ocular reflec arc. Arch Neurol Psychiatry 30:245–291
Mathew PJ, Madan R, Subramaniam R, Bhatia A, Mala CG, Soodan A, Kaul HL (2004) Efficacy of low-dose dexamethasone for preventing postoperative nausea and vomiting following strabismus repair in children. Anaesth Intensive Care 32:372–376
Matsnev EI, Yakovleva IY, Tarasov IK, Alekseev VN, Kornilova LN, Mateev AD, Gorgiladze GI (1983) Space motion sickness: phenomenology, countermeasures, and mechanisms. Aviat Space Environ Med 54:312–317
Matsuo V, Cohen B (1984) Vertical optokinetic nystagmus and vestibular nystagmus in the monkey: up-down asymmetry and effects of gravity. Exp Brain Res 53:197–216
Miller EF, Graybiel A (1969) A standardized laboratory means of determining susceptibility to coriolis (motion) sickness. Aerospace Medical Institute NAMI-1058
Money KE (1972) Motion sickness. Physiol Rev 50:1–39
Money KE (1981) Biological effects of space travel. Can Aeronaut Space J 27:195–201
Nachum Z, Gordon CR, Shahal B, Spitzer O, Shupak A (2002) Active high-frequency vestibulo-ocular reflex and seasickness susceptibility. Laryngoscope 112:179–182
Newlands SD, Dara S, Kaufman GD (2005) Relationship of static and dynamic mechanisms in vestibuloocular reflex compensation. Laryngoscope 115:191–204
Palla A, Straumann D (2004) Recovery of the high-acceleration vestibulo-ocular reflex after vestibular neuritis. J Assoc Res Otolaryngol 5:427–435
Putcha L, Berens KL, Marshburn TH, Ortega HJ, Billica RD (1999) Pharmaceutical use by US astronauts on space shuttle missions. Aviat Space Environ Med 70:705–708
Raphan T, Cohen B (1981) The role of integration in oculomotor control. In: Zuber BL (ed) Models of Oculomotor Behavior and Control. CRC Press, Boca Raton, Fla., pp 91–109
Raphan T, Cohen B (1988) Organizational principles of velocity storage in three dimensions: The effect of gravity on cross-coupling of optokinetic after-nystagmus. Ann NY Acad Sci 545:74–92
Raphan T, Cohen B (2002) The vestibulo-ocular reflex (VOR) in three dimensions. Exp Brain Res 145:1–27
Raphan T, Sturm D (1991) Modelling the spatiotemporal organization of velocity storage in the vestibuloocular reflex by optokinetic studies. J Neurophysiol 66:1410–1420
Raphan T, Dai M, Cohen B (1992) Spatial orientation of the vestibular system. Ann NY Acad Sci 656: 140–157
Raphan T, Matsuo V, Cohen B (1979) Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res 35:229–248
Reason JT, Brand JJ (1975) Motion sickness. Academic, London
Reisine H, Raphan T (1992) Neural basis for eye velocity generation in the vestibular nuclei during off-vertical axis rotation. Exp Brain Res 92:209–226
Schnabolk C, Raphan T (1992) Modelling 3-D slow phase velocity estimation during off-vertical axis rotation (OVAR). J Vest Res 2:1–14
Sheliga BM, Yakushin SB, Silvers A, Raphan T, Cohen B (1999) Control of spatial orientation of the angular vestibulo-ocular reflex by the nodulus and uvula of the vestibulocerebellum. Ann NY Acad Sci 871:94–122
Shupak A, Kerem D, Gordon C, Spitzer O, Mendelowitz N, Melamed Y (1990) Vestibulo-ocular reflex as a parameter of seasickness susceptibility. Ann Otol Rhinol Laryngol 99:131–136
Singleton GT (1967) Relationships of the cerebellar nodulus to vestibular function: a study of the effects of nodulectomy on habituation. Laryngoscope 77:1579–1620
Solomon D, Cohen B (1994) Stimulation of the nodulus and uvula discharges velocity storage in the vestibulo-ocular reflex. Exp Brain Res 102:57–68
Thornton WE, Uri JJ (1991) Oculomotor function during space flight and susceptibility to space motion sickness. Acta Astronaut 23:53–61
Torte MP, Clément G, Courjon JH, Magenes G (1997) Absence of habituation of the vertical vestibulo-ocular reflex in the cat. Exp Brain Res 116:73–82
Uemura T, Cohen B (1973) Effects of vestibular nuclei lesion on vestibulo-ocular reflexes and posture in monkeys. Acta Otolaryngol Suppl 315:1–71
Wade SW, Halmagyi GM, Black FO, McGarvie LA (1999) Time constant of nystagmus slow-phase velocity to yaw-axis rotation as a function of the severity of unilateral caloric paresis. Am J Otol 20:471–478
Waespe W, Cohen B, Raphan T (1985) Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science 228:199–201
Wang SC, Chinn HI (1956) Experimental motion sickness in dogs. Importance of labyrinth and vestibular cerebellum. Am J Physiol 185:617–623
Wearne S, Raphan T, Cohen B (1998) Control of spatial orientation of the angular vestibuloocular reflex by the nodulus and uvula. J Neurophysiol 79:2690–2715
Yakushin SB, Raphan T, Cohen B (2000) Context-specific adaptation of the vertical vestibuloocular reflex with regard to gravity. J Neurophysiol 84:3067–3071
Yardley L, Lerwill H, Hall M, Gresty M (1992) Visual destabilisation of posture in normal subjects. Acta Otolaryngol 112:14–21
Yates BJ (1992) Vestibular influences on the sympathetic nervous system. Brain Res Brain Res Rev 17:51–59
Yates BJ, Miller AD (1998) Physiological evidence that the vestibular system participates in autonomic and respiratory control. J Vestib Res 8:17–25
Yokota JI, Reisine H, Cohen B (1992) Nystagmus induced by electrical microstimulation of the vestibular and prepositus hypoglossi nuclei in the monkey. Exp Brain Res 92:123–138
Young LR, Hecht H, Lyne L, Sienko K, Cheung C, Kavelaars J (2001) Artificial gravity: head movements during short-radius centrifugation. Acta Astronaut 49:215–226
Acknowledgments
This work was supported by NASA NCC 9-58-25, NSBRI NA00406, NIDCD DC5222, NIDCD DC05204, and EY01867.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, M., Raphan, T. & Cohen, B. Labyrinthine lesions and motion sickness susceptibility. Exp Brain Res 178, 477–487 (2007). https://doi.org/10.1007/s00221-006-0759-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0759-1