Abstract
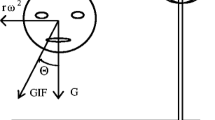
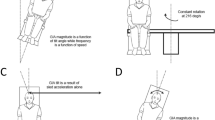
Tilting the head in roll to or from the upright while rotating at a constant velocity (roll while rotating, RWR) alters the position of the semicircular canals relative to the axis of rotation. This produces vertical and horizontal nystagmus, disorientation, vertigo, and nausea. With recurrent exposure, subjects habituate and can make more head movements before experiencing overpowering motion sickness. We questioned whether promethazine lessened the vertigo or delayed the habituation, whether habituation of the vertigo was related to the central vestibular time constant, i.e., to the time constant of velocity storage, and whether the severity of the motion sickness was related to deviation of the axis of eye velocity from gravity. Sixteen subjects received promethazine and placebo in a double-blind, crossover study in two consecutive 4-day test series 1 month apart, termed series I and II. Horizontal and vertical eye movements were recorded with video-oculography while subjects performed roll head movements of approx. 45° over 2 s to and from the upright position while being rotated at 138°/s around a vertical axis. Motion sickness was scaled from 1 (no sickness) to an endpoint of 20, at which time the subject was too sick to continue or was about to vomit. Habituation was determined by the number of head movements that subjects made before reaching the maximum motion sickness score of 20. Head movements increased steadily in each session with repeated testing, and there was no difference between the number of head movements made by the promethazine and placebo groups. Horizontal and vertical angular vestibulo-ocular reflex (aVOR) time constants declined in each test, with the declines being closely correlated to the increase in the number of head movements. The strength of vertiginous sensation was associated with the amount of deviation of the axis of eye velocity from gravity; the larger the deviation of the eye velocity axis from gravity, the more severe the motion sickness. Thus, promethazine neither reduced the nausea associated with RWR, nor retarded or hastened habituation. The inverse relationship between the aVOR time constants and number of head movements to motion sickness, and the association of the severity of motion sickness with the extent, strength, and time of deviation of eye velocity from gravity supports the postulate that the spatiotemporal properties of velocity storage, which are processed between the nodulus and uvula of the vestibulocerebellum and the vestibular nuclei, are likely to represent the source of the conflict responsible for producing motion sickness.







Similar content being viewed by others
References
Arai Y, Yakushin SB, Suzuki J-I, Cohen B, Raphan T (2002) Spatial orientation of caloric nystagmus in canal plugged monkeys. J Neurophysiol 88:914–928
Bard P (1945) Committee on aviation medicine. Report No. 485, National Research Council
Barmack NH, Baughman RW, Eckenstein FP, Shojaku H (1992) Secondary vestibular cholinergic projection to the cerebellum of rabbit and rat as revealed by choline acetyltransferase immunohistochemistry, retrograde and orthograde tracers. J Comp Neurol 317:250–270
Benson AJ, Bodin MA (1966) Interaction of linear and angular accelerations on vestibular receptors in man. Aerosp Med 37:144–154
Bles W (1988) Coriolis effects and motion sickness modeling. Brain Res Bull 47:543–549
Bles W, Bos JE, Graaf B de, Groen E, Wertheim AH (1998) Motion sickness: only one provocative conflict? Brain Res Bull 47:481–487
Bos JE, Bles W, Graaf B de (2002) Eye movements to yaw, pitch, and roll about vertical and horizontal axes: adaptation and motion sickness. Aviat Space Environ Med 73:436–434
Brandt T, Wist E, Dichgan J (1971) Optisch induzierte pseudocoriolis-effekten und circularvektion. Arch Psych Nervenkrank 214:365–389
Büttner U, Waespe W (1981) Vestibular nerve activity in the alert monkey during vestibular and optokinetic nystagmus. Exp Brain Res 41:310–315
Cheung BS, Howard IP, Money KE (1991) Visually induced sickness in normal and bilaterally labyrinthine-defective subjects. Aviat Space Environ Med 62:527–531
Clément G, Deguine O, Parant M, Costes-Salon MC, Vasseur-Clausen P, Pavy-LeTraon A (2001) Effects of cosmonaut vestibular training on vestibular function prior to space flight. Eur J Appl Physiol 85:539–545
Cobb GW (1998) Design and analysis of experiments. Springer, New York
Cohen B, Uemura T, Takemori S (1973) Effects of labyrinthectomy on optokinetic nystagmus (OKN) and optokinetic after-nystagmus (OKAN). Equilib Res 3:88–93
Cohen B, Matsuo V, Raphan T (1977) Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol (Lond) 270:321–344
Cohen B, John P, Yakushin SB, Buettner-Ennever J, Raphan T (2002) The nodulus and uvula; source of cerebellar control of spatial orientation of the angular vestibulo-ocular reflex. Ann NY Acad Sci 978:28–45
Cohen H, Cohen B, Raphan T, Waespe W (1992) Habituation and adaptation of the vestibulo-ocular reflex: a model of differential control by the vestibulo-cerebellum. Exp Brain Res 90:526–538
Collins WE, Schroeder DJ, Elam GW (1982) A comparison of some effects of three antimotion sickness drugs on nystagmus responses to angular accelerations and to optokinetic stimuli. Aviat Space Environ Med 53:1182–1189
Dai M, Raphan T, Cohen B (1991) Spatial orientation of the vestibular system: dependence of optokinetic after nystagmus on gravity. J Neurophysiol 66:1422–1438
Dai M, Klein A, Cohen B, Raphan T (1999) Model-based study of the human cupular time constant. J Vest Res 9:293–301
Dai M, Kaufmann H, Raphan T, Cohen B (2000) Promethazine affects optokinetic but not vestibular responses in monkeys. Aviat Space Environ Med 71:1003–1012
Davis JR, Vanderploeg JM, Santy PA, Jenings RT, Stewart DF (1988) Space motion sickness during 24 flights of the space shuttle. Aviat Space Environ Med 59:1185–1189
Deane FR, Wood CD, Graybiel A (1967) The effect of drugs in altering susceptibility to motion sickness in aerobatics and the slow rotation room. Aerosp Med 38:842–845
DiZio P, Lackner JR (1988) The effects of gravitoinertial force level and head movements on postrotational nystagmus and illusory after-rotation. Exp Brain Res 70:485–495
DiZio P, Lackner JR (1991) Motion sickness susceptibility in parabolic flight and velocity storage activity. Aviat Space Environ Med 62:300–307
Ebenholtz SM (1992) Motion sickness and oculomotor systems in virtual environments. Presence 1:302–305
Gizzi M, Raphan T, Rudolph S, Cohen B (1994) Orientation of human optokinetic nystagmus to gravity: a model based approach. Exp Brain Res 99:347–360
Goldberg JM, Fernandez C (1971) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to angular accelerations. J Neurophysiol 34:635–660
Graaf B de, Bles W, Bos JE (1999) Roll motion stimuli: sensory conflict, perceptual weighting and motion sickness. Brain Res Bull 47:489–495
Graybiel A, Knepton J (1977) Evaluation of a new antinauseant drug for the prevention of motion sickness. Aviat Space Environ Med 48:867–471
Graybiel A, Lackner JR (1987) Treatment of severe motion sickness with antimotion sickness drug injections. Aviat Space Environ Med 58:773–776
Graybiel A, Miller EF, Homick JL (1977) Experiment M131: human vestibular function. In: Johnston RS, Dietlein LF (eds) Biomedical results from Skylab, vol SP-377. Scientific and Technical Information Office, NASA, Washington, DC, pp 74–133
Grüsser O-J (1984) J. E. Purkinjě's contributions to the physiology of the visual, the vestibular and the oculomotor system. Hum Neurobiol 3:129–144
Guedry FE (1965) Habituation to complex vestibular stimulation in man: transfer and retention of effects from twelve days of rotation at 10 rpm. Percept Mot Skills 21:459–481
Guedry FE (1974) Psychophysics of vestibular sensation. In: Kornhuber HH (ed) Handbook of sensory physiology, vol 6. Springer, Berlin, Heidelberg, New York, pp 3–154
Guedry FE, Benson AJ (1978) Coriolis crosscoupling effects: disorienting and nauseogenic or not? Aviat Space Environ Med 49:29–35
Guedry FE, Graybiel A, Collins WE (1962) Reduction of nystagmus and disorientation in human subjects. Aerospace Med 33:1356–1360
Guedry FE, Collins WE, Graybiel A (1964) Vestibular habituation during repetitive complex stimulation: a study of transfer effects. J Appl Physiol 19:1005–15
Guedry FE, Rupert AR, Reschke MF (1998) Motion sickness and development of synergy within the spatial orientation system. A hypothetical unifying concept. Brain Res Bull 47:475–480
Hecht H, Kavelaars J, Cheung CC, Young LR (2001) Orientation illusions and heart-rate changes during short-radius centrifugation. J Vest Res 11:115–127
Howard IP, Templeton WB (1966) Human spatial orientation. Wiley, New York
Kellogg RS, Kennedy RS, Graybiel A (1965) Motion sickness symptomatology of labyrinthine defective and normal subjects during zero gravity maneuvers. Aerosp Med 36:315–318
Kennedy RS, Graybiel A (1962) Validity of tests of canal sickness in predicting susceptibility to airsickness and seasickness. Aerosp Med 33:935–938
Kennedy RS, Graybiel A, McDonough RG, Beckwith RD (1968) Symptomatology under storm conditions in the North Atlantic in control subjects and in person with bilateral labyrinthine defects. Acta Otolaryngol (Stockh) 66:533–540
Lackner JR, Graybiel A (1980) Elicitation of motion sickness by head movements in the microgravity phase of parabolic flight maneuvers. Aviat Space Environ Med 55:513–520
Lackner JR, Graybiel A (1986) The effective intensity of Coriolis, crosscoupling stimulation is gravitoinertial force dependent: implications for space motion sickness. Aviat Space Environ Med 57:229–235
Lackner JR, Graybiel A (1994) Use of promethazine to hasten adaptation to provocative motion. J Clin Pharmacol 34:644–648
Matsuo V, Cohen B (1984) Vertical optokinetic nystagmus and vestibular nystagmus in the monkey: Up-down asymmetry and effects of gravity. Exp Brain Res 53:197–216
McCabe BE (1960) Vestibular suppression in figure skaters. Trans Am Acad Ophthalmol Otol 64:264–268
Miller AD, Wilson VJ (1983) Vestibular-induced vomiting after vestibulocerebellar lesions. Brain Behav Evol 23:26–31
Miller EF, Graybiel A (1973) Experiment M-131—Human vestibular function. Aerospace Med 44:593–608
Money KE (1972) Motion sickness. Physiol Rev 50:1–39
Oman CM (1982) A heuristic mathematical model for the dynamics of sensory conflict and motion sickness. Acta Otolaryngol (Suppl) 392:4–44
Oman CM, Balkwill MD (1993) Horizontal angular VOR, nystagmus dumping, and sensation duration in Spacelab SLS-1 crewmembers. J Vest Res 3:315–330
Peterka RJ, Black FO, Schoenhoff MB (1987) Optokinetic and vestibulo-ocular reflex responses to an unpredictable stimulus. Aviat Space Environ Med 58:180–185
Purkinjě JE (1820) Beiträge zur näheren Kenntnis des Schwindels aus heutognostischen Daten. Med JB (Wien) 6:79–125
Quarck G, Etard O, Oreel M, Denise P (2000) Motion sickness occurrence does not correlate with nystagmus characteristics. Neurosci Lett 287:49–52
Raphan T, Cohen B (1988) Organizational principles of velocity storage in three dimensions: The effect of gravity on crosscoupling of optokinetic after-nystagmus. Ann NY Acad Sci 545:74–92
Raphan T, Cohen B (2002) The vestibulo-ocular reflex (VOR) in three dimensions. Exp Brain Res 145:1–27
Raphan T, Sturm D (1991) Modelling the spatiotemporal organization of velocity storage in the vestibulo-ocular reflex by optokinetic studies. J Neurophysiol 66:1410–1420
Raphan T, Matsuo V, Cohen B (1979) Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res 35:229–248
Raphan T, Dai M, Cohen B (1992) Spatial orientation of the vestibular system. Ann NY Acad Sci 656:140–157
Raphan T, Wearne S, Cohen B (1996) Modeling the organization of the linear and angular vestibulo-ocular reflexes. Ann NY Acad Sci 781:348–363
Reason JT, Brand JJ (1975) Motion sickness. Academic, London
Schrader V, Koenig E, Dichgans J (1985a) Direction and angle of active head tilts influencing the Purkinjě effect and the inhibition of postrotatory nystagmus I and II. Acta Otolaryngol (Stockh) 100:337–343
Schrader V, Koenig E, Dichgans J (1985b) The effect of lateral head tilt on horizontal postrotatory nystagmus I and II and the Purkinjě effect. Acta Otolaryngol (Stockh) 100:98–105
Schroeder DJ, Collins WE, Elam GW (1985) Effects of some motion sickness suppressants on static and dynamic tracking performance. Aviat Space Environ Med 56:344–350
Sheliga BM, Yakushin SB, Silvers A, Raphan T, Cohen B (1999) Control of spatial orientation of the angular vestibulo-ocular reflex by the nodulus and uvula of the vestibulocerebellum. Ann NY Acad Sci 871:94–122
Singleton GT (1967) Relationships of the cerebellar nodulus to vestibular function: a study of the effects of nodulectomy on habituation. Laryngoscope 77:1579–1620
Solomon D, Cohen B (1992) Stabilization of gaze during circular locomotion in darkness; II. Contribution of velocity storage to compensatory eye and head nystagmus in the running monkey. J. Neurophysiol 67:1158–1170
Solomon D, Cohen B (1994) Stimulation of the nodulus and uvula discharges velocity storage in the vestibulo-ocular reflex. Exp Brain Res 102:57–68
Triesman M (1977) Motion sickness: an evolutionary hypothesis. Science 197:493–495
Tweed D, Fetter M, Sievering D, Misslisch H, Koenig E (1994a) Rotational kinematics of the human vestibulo-ocular reflex II. Gain matrices. J Neurophysiol 72:2480–2489
Tweed D, Sievering D, Misslisch H, Fetter M, Zee D, Koenig E (1994b) Rotational kinematics of the human vestibulo-ocular reflex I. Gain matrices. J Neurophysiol 72:2467–2479
Tyler DB, Bard P (1949) Motion sickness. Physiol Rev 29:311–369
Waespe W, Cohen B, Raphan T (1985) Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science 228:199–201
Wang SC, Chinn HI (1956) Experimental motion sickness in dogs. Importance of labyrinth and vestibular cerebellum. Am J Physiol 185:617–623
Wearne S, Raphan T, Cohen B (1996) Nodulo-uvular control of central vestibular dynamics determines spatial orientation of the angular vestibulo-ocular reflex (aVOR). Ann NY Acad Sci 781:364–384
Wearne S, Raphan T, Cohen B (1998) Control of spatial orientation of the angular vestibulo-ocular reflex by the nodulus and uvula. J Neurophysiol 79:2690–2715
Wood CD, Graybiel A (1968) Evaluation of sixteen anti-motion sickness drugs under controlled laboratory conditions. Aerosp Med 39:1341–1344
Wood CD, Manno JE, Manno BR, Redetzki HM, Wood M, Vekovius WA (1984) Side effects of antimotion sickness drugs. Aviat Space Environ Med 55:113–116
Wood CD, Manno JE, Redetzki HM, Wood MJ, Mims ME (1985) Evaluation of antimotion sickness drug side effects on performance. Aviat Space Environ Med 56:310–316
Yates BJ, Miller AD (1998) Physiological evidence that the vestibular system participates in autonomic and respiratory control. J Vest Res 8:17–25
Yates BJ, Miller AD, Lucot JD (1998) Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull 47:395–406
Young LR (1999) Artificial gravity consideration for a Mars exploration mission. Ann NY Acad Sci 871:367–378
Young LR, Hecht H, Lyne L, Sienko K, Cheung C, Kavelaars J (2001) Artificial gravity: head movements during short-radius centrifugation. Acta Astronautica 49:215–226
Acknowledgements
The authors are grateful to the subjects for their dedication and endurance throughout the experiments. Thanks are also extended to Drs. Laurence Young and Heiko Hecht of MIT for sharing their expertise on production and coding of motion sickness. The authors were supported by NSBRI NCC 9-58-25 (M.D.), NSBRI NCC9-58 (T.R.), DC05222 (T.R.), DC03284 (B.C.), DC05204 (B.C.), and EY01867.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, M., Kunin, M., Raphan, T. et al. The relation of motion sickness to the spatial–temporal properties of velocity storage. Exp Brain Res 151, 173–189 (2003). https://doi.org/10.1007/s00221-003-1479-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1479-4