Abstract
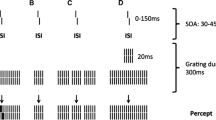
The aim of two experiments was to investigate the relationship between spatio-temporal contrast sensitivity and visual backward masking in normal observers and in subgroups with positive or negative symptoms in schizophrenia. Experiment 1 measured contrast sensitivity for stationary and counterphase-modulated sinusoidal gratings at four spatial (0.5, 2.0, 4.0, 8.0 cycles/degree) and four temporal frequencies (0, 4.0, 8.0, 16.0 Hz). The results showed that there were no differences in spatio-temporal contrast sensitivity between the control and positive-symptom group, and in comparison with these groups, contrast sensitivity was significantly lower at all spatial and temporal frequencies in the negative-symptom group. Experiment 2 measured the visibility of a Landolt C target with a constant target stimulus duration of 4.0 ms followed by a 150-ms backward mask, which was presented at 12 stimulus onset asynchronies from 0 to 110 ms in the same groups of observers. Consistent with the findings of the previous experiment, there were no significant differences in backward masking between the control and positive-symptom group, and in comparison with these groups, visual backward masking was significantly higher at all stimulus onset asynchronies from 40 to 110 ms in the negative-symptom group. The present findings show that there were no significant differences in contrast sensitivity and in backward masking between normal observers and a group with positive symptoms in schizophrenia. It was concluded that the reduction in contrast sensitivity for low spatial frequency counterphase flicker in the negative-symptom group is consistent with a reduction in the ‘contrast gain control’ mechanism of magnocellular channels, and that the reduction in contrast sensitivity for medium and high stationary gratings is consistent with a disorder in parvocellular channels. It was proposed that a disorder in magnocellular channels in the negative-symptom group may enforce a reliance on parvocellular channels that results in longer temporal summation and visible persistence, slower visual processing of single target stimuli at threshold and higher levels of sensory integration, and backward masking when multiple stimuli are presented rapidly in time.




Similar content being viewed by others
References
Allport DA (1970) Temporal summation and phenomenal simultaneity: experiments with the radius display. Q J Exp Psychol 22:686–701
American Psychiatric Association (1992) Diagnostic and statistical manual of mental disorders, DSM-IV, 4th edn. American Psychiatric Association, Washington DC
Anderson JE, Wible CG, McCarley RW, Jacab M, Kasai K, Shenton ME (2002) An MRI study of temporal lobe abnormalities and negative symptoms in chronic schizophrenia. Schizophr Res 58:123–134
Andreasen NC (1981) Scale for the assessment of negative symptoms (SANS). University of Iowa, Iowa City
Andreasen NC (1983) Scale for the Assessment of Positive symptoms (SANS). University of Iowa, Iowa City
Andreasen NC (1990) Positive and negative symptoms: Historical and conceptual aspects. In: Andreasen NC (ed) Schizophrenia: positive and negative symptoms and syndromes. Karger, Basel, pp 1–42
Andreasen NC, Olsen SA (1982) Negative, positive schizophrenia: definition and validation. Arch Gen Psychiatry 39:789–795
Andreasen NC, Flaum M, Arndt S, Allinger R, Swayze VW (1991) Positive and negative symptoms: assessment and validity. In: Marneros A, Andreasen NC, Tsuang MT (eds) Negative versus positive schizophrenia. Springer-Verlag, New York, Berlin, Heidelberg
Balogh DW, Merritt RD (1987) Visual masking and the schizophrenia spectrum: interfacing clinical and experimental methods. Schizophr Bull 13:679–698
Basso MR, Nasrallah HA, Olsen SC, Bornstein RA (1988) Neuropsychological correlates of negative, disorganised and psychotic symptoms in schizophrenia. Schizophr Res 31:99–111
Benardete EA, Kaplan E, Knight BW (1992) Contrast gain control in primate retina: P cells are not X-like, some M cells are. Vis Neurosci 8:483–486
Bodis-Wollner I (1972) Visual acuity and contrast sensitivity in patients with cerebral lesions. Science 178:769–771
Bodis-Wollner I, Tzelepi A (1998) The push-pull action of dopamine on spatial tuning of the monkey retina: the effects of dopaminergic deficiency and selective D1 and D2 receptor ligands on the pattern electroretinogram. Vision Res 38:1479–1487
Bowling A, Lovegrove WJ (1980) The effect of spatial frequency on the persistence of gratings. Percept Psychophys 27:574–578
Braff DL (1981) Early information processing deficit in schizophrenia. Arch Gen Psychiatry 38:175–179
Braff DL (1989) Sensory input deficits and negative symptoms in schizophrenia patients. Am J Psychiatry 146:1006–1011
Braff DL, Saccuzzo DP (1981) Information processing dysfunction in paranoid schizophrenia: a two-factor deficit. Am J Psychiatry 138:1051–1056
Braff DL, Saccuzzo DP (1982) Effects of antipsychotic medication on speed of information processing in schizophrenic patients. Am J Psychiatry 139:1127–1130
Braff DL, Heaton R, Kuck J, Cullum M, Moranville J, Grant I, Zisook S (1991) The generalised pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Arch Gen Psychiatry 48:891–898
Breitmeyer BG (1980) Unmasking visual masking: a look at the ‘why’ behind the veil of the ‘how’. Psychol Rev 87:52–69
Breitmeyer BG (1984) Visual masking: an integrative approach. Clarendon Press, Oxford
Breitmeyer BG (1992) Parallel processing in human vision: history, review and critique. In: Brannan JR (ed) Applications of parallel processing in vision. North-Holland, Amsterdam, pp 37–38
Breitmeyer BG, Ganz L (1976) Implications of sustained and transient channels for theories of visual pattern masking, saccadic suppression, and information processing. Psychol Rev 83:1–36
Breitmeyer BG, Ogmen H (2000) Recent models and findings in visual backward masking: a comparison, review, and update. Percept Psychophys 62:1572–1595
Buchanan RW, Kirkpatrick B, Heinrichs DW (1990) Clinical correlates of the deficit syndrome of schizophrenia. Am J Psychiatry 147:290–294
Buchanan RW, Breier A, Kirkpatrick B (1993) Structural abnormalities in deficit and nondeficit schizophrenia. Am J Psychiatry 150:59–65
Burr DC, Ross J (1982) Contrast sensitivity at high velocities. Vision Res 23:3567–3569
Butler PD, Harkavy-Friedman JM, Amador XF, Gorman JM (1996) Backward masking in schizophrenia: relationship to medication status, neuropsychological function, and dopamine metabolism. Biol Psychiatry 40:295–298
Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC (2001) Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry 158:1126–1133
Butler PD, DeSanti LA, Maddox J, Harkavy-Friedman JM, Amador XF, Goetz RR, Javitt DC, Gorman JM (2002) Visual backward masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophr Res 59:199–209
Cadenhead KS, Geyer MA, Butler RW, Perry W, Sprock J, Braff DL (1997) Information processing deficits of schizophrenia patients: relationship to clinical ratings, gender and medication status. Schizophr Res 28:51–62
Carpenter WT, Heinrichs DW, Wagman AM (1988) Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry 145:578–583
Coltheart M (1980) Iconic memory and visible persistence. Percept Psychophys 27:183–228
Crow TJ (1982) Positive and negative symptoms and the role of dopamine in schizophrenia. In: Hemmings G (ed) Biological aspects of schizophrenia. John Wiley & Sons, New York
Crow TJ (1985) The two-syndrome concept: origins and current status. Schizophr Bull 11:471–486
Crow TJ (1995) Brain changes in negative symptoms in schizophrenia. Psychopathology 28:18–21
De Valois RL, De Valois KK (1990) Spatial vision. Oxford University Press, New York
De Valois RL, De Valois KK (1993) A multi-stage colour model. Vision Res 33:1053–1065
Derrington AM, Lennie P (1984) Spatial and temporal contrast sensitivities of neurones in the lateral geniculate nucleus of the macaque. J Physiol 357:219–240
Diefendorf AR, Dodge R (1908) An experimental study of the ocular reactions of the insane from photographic records. Brain 31:451–489
Dobkins KR, Gunther KL, Peterzell DH (2000) What covariance mechanisms underlie green/red equiluminance, luminance contrast sensitivity and chromatic (red/green) contrast sensitivity? Vision Res 40:613–628
Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC (2002) Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry 59:1011–1020
Efron R (1970a) The relationship between the duration of a stimulus and the duration of a perception. Percept Psychophys 8:37–55
Efron R (1970b) Effect of stimulus duration on perceptual onset and offset latencies. Percept Psychophys 8:231–234
Enroth-Cugell CA, Robson JG (1966) The contrast sensitivity of retinal ganglion cells of the cat. J Physiol 187:517–552
Finlay GA (1985) High-speed point plotter for vision research. Vision Res 25:1993–1997
Ganz L (1975) Temporal factors in perception. In: Carterette FC, Friedman MP (eds) Handbook of perception, vol 5. Seeing. Academic Press, New York
Gerbaldo H, Philipp M (1995) The deficit syndrome in schizophrenic and nonschizophrenic patients: preliminary studies. Psychopathology 28:55–63
Gerbaldo H, Thaker G, Titel P (1992) Abnormal electroretinography in schizophrenic patients with a history of sun gazing. Neuropsychobiology 2:99–101
Green DM, Swets JA (1966) Signal detection theory and psychophysics. Wiley, New York
Green M, Walker E (1984) Susceptibility to backward masking in schizophrenic patients with positive and negative symptoms. Am J Psychiatry 141:1273–1275
Green M, Walker E (1986) Symptom correlates of vulnerability to backward masking in schizophrenia. Am J Psychiatry 143:181–186
Green MF, Neuchterlein KH, Mintz J (1994a) Backward masking in schizophrenia and mania. I. Specifying a mechanism. Arch Gen Psychiatry 51, 939–944
Green MF, Neuchterlein KH, Mintz J (1994b) Backward masking in schizophrenia and mania. II. Specifying the visual channels. Arch Gen Psychiatry 51:945–951
Green MF, Mintz J, Salveson D, Neuchterlein KH, Breitmeyer B, Light GA, Braff DL (2003) Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biol Psychiatry 53:1113–1119
Haber RN, Standing L (1969) Direct measures of the apparent duration of a flash. Can J Psychol 24:216–229
Hacker MJ, Ratcliff R (1979) A revised table for d′ for M-alternative forced choice. Percept Psychophys 26:168–170
Harris JP, Calvert JE, Leendertz JA, Phillipson OT (1990) The influence of dopamine on spatial vision. Eye 4:806–812
Holzman PS, Proctor LR, Hughes DW (1973) Eye tracking pattern in schizophrenia. Science 81:179–181
Hood DC (1998) Lower-level processing and models of light adaptation. Annu Rev Psychol 49:503–535
Kaplan E, Shapley RM (1986) The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc Natl Acad Sci 38:2755–2757
Kaplan E, Lee BB, Shapley RM (1990) New views of primate retinal function. In: Osborne N, Chader GD (eds) Progress in retinal research, vol 9. Pergamon, Oxford, pp 273–336
Katcher BS, Young LY, Koda-Kimble MA (1988) Applied therapeutics: the clinical use of drugs, 4th edn. Applied Therapeutics, Vancouver
Katsanis J, Iacono W (1991) Clinical, neuropsychological, and brain structure correlates of smooth pursuit eye movement performance in chronic schizophrenics. J Abnorm Psychol 100:526–534
Kay SR (1991) Positive and negative syndromes in schizophrenia: assessment and research. Brunner/Mazel, New York
Keefe R, Siever L, Mohs R, Peterson AE, Mahon, TR, Berman RL, Davis, KL (1989) Eye tracking, schizophrenic symptoms, and schizotypal personality disorder. Eur Arch Psychiatry Neurol Sci 239:39–42
Keilp JG, Sweeney JA, Jacobsen P, Solomon C, St Luois L, Deck M, Frances A, Mann JJ (1988) Cognitive impairments in schizophrenia: specific relations to ventricular size and negative symptomatology. Biol Psychiatry 24:47–55
Kelly DH (1977) Visual contrast sensitivity. Optica Acta 24:107–129
Keri S, Antal A, Szekeres G, Benedek G, Janka Z (2000) Visual information processing in patients with schizophrenia: evidence for the impairment of central mechanisms. Neurosci Lett 293:69–71
Keri S, Antal A, Szekeres G, Benedek G, Janka Z (2002) Spatiotemporal processing in schizophrenia. J Neuropsychiatry Clin Neurosci 14:190–196
Kolers PA (1962) Intensity and contour effects in visual masking. Vision Res 2:277–294
Kulikowski JJ, Tolhurst DJ (1973) Psychological evidence for sustained and transient detectors in human vision. J Physiol 232:149–162
Lamme VA, Zipster K, Spekreijse H (2002) Masking interrupts figure-ground signals in V1. J Cogn Neurosci 14:1044–1053
Lee BB, Pokorny J, Smith VC, Martin PR, Valberg A (1990) Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. J Opt Soc Am 7:2223–2236
Legge G (1978) Sustained and transient mechanisms in human vision: temporal and spatial properties. Vision Res 18:69–81
Lennie P (1980) Visual pathways: a review. Vision Res 20:561–594
Lewis DA, Levitt P (2002) Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 25:409–432
Liddle PF (1987) The symptoms of chronic schizophrenia. A re-examination of the positive-negative dichotomy. Br J Psychiatry 151:145–151
Lorr M, McNair DM, Klett CJ, Lasky JJ (1962) Evidence of ten psychotic syndromes. J Consult Clin Psychol 26:185–189
Maunsell JHR (1992) Functional visual streams. Curr Opin Neurobiol 2:506–510
Merigan WH, Eskin TA (1986) Spatio-temporal vision of macaques with severe loss of PΒ retinal ganglion cells. Vision Res 26:1751–1761
Merigan WH, Maunsell JHR (1990) Macaque vision after magnocellular lateral geniculate lesions. Vis Neurosci 5:347–352
Merigan WH, Maunsell JHR (1993) How parallel are the primate visual pathways? Annu Rev Neurosci 16:369–402
Merriam AE, Kay, SR, Weiner RU, Opler LA (1990) Negative schizophrenic symptoms are associated with backward masking vulnerability. Schizophr Res 3:355–356
Milner AD, Goodale MA (1995) The Visual Brain In Action. Oxford University Press, Oxford
Muise J, LeBlanc RS, Lavoie ME, Arsenault AS (1991) Two-stage model of visual masking: sensory transmission and accrual of effective information as a function of target intensity and similarity. Percept Psychophys 50:197–204
Nelson HE (1983) National adult reading test (NART). NFER-Nelson Publishing, Windsor
O’Donnell BF, Stylianopoulus KC, Swearer JM, McCarley RW (1987) Spatial frequency discrimination in schizophrenia. Biol Psychiatry 41:1S–120S
Ott SL, Roberts S, Rock D, Allen J, Erlenmeyer-Kimling L (2002) Positive and negative thought disorder and psychopathology in childhood among subjects with adult schizophrenia. Schizophr Res 58:231–239
Overall JE, Gorham DR (1962) Brief psychiatric rating scale. Psychol Rep 10:799–912
Owsley C, Sekuler R, Siemsen D (1983) Contrast sensitivity throughout adulthood. Vision Res 23, 689–699
Ross DE, Thaker GK, Buchanan RW, Lahti AC, Medoff D, Bartko JJ, Moran M, Hartley J (1996) Association of abnormal smooth pursuit eye movements with the deficit syndrome in schizophrenic patients. Am J Psychiatry 153:1158–1165
Ross DE, Thaker GK, Buchanan RW, Kirkpatrick B, Lahti AC, Medoff D, Bartko JJ, Goodman J, Tien A (1997) Eye tracking disorder in schizophrenia is characterised by specific oculomotor defects and is associated with the deficit syndrome. Biol Psychiatry 42:781–796
Saccuzzo DP, Braff DL (1981) Early information processing deficit in schizophrenia: New findings using schizophrenic subgroups and manic control subjects. Arch Gen Psychiatry 38:175–179
Saccuzzo DP, Hirt M, Spencer TJ (1974) Backward masking as a measure of attention in schizophrenia. J Abnorm Psychol 83:512–522
Sallet PC, Elkis H, Alves TM, Oliveira JR, Sassi E, de Castro CC, Busatto GF, Gattaz WF (2003) Rightward cerebral asymmetry in subtypes of schizophrenia according to Leonhard’s classification and to DSM-IV: a structural MRI study. Psychiatry Res Neuroimaging 123:65–79
Sawatari A, Callaway EM (1996) Convergence of magno- and parvocellular pathways in layer 4b of macaque primary visual cortex. Nature 380:442–446
Scheerer E (1973) Integration, interruption and processing rate in visual backward masking. Psychol Forschung 36:71–93
Schiller PH, Malpeli JG (1978) Properties and tectal projections of monkey ganglion cells. J Neurophysiol 40:428–445
Schuck JR, Lee RG (1989) Backward masking, information processing, and schizophrenia. Schizophr Bull 15:491–500
Schwartz BD, Winstead DK (1982) Visual processing deficits in acute and chronic schizophrenics. Biol Psychiatry 17:1377–1387
Schwartz BD, Winstead DK (1985) Icon formation in chronic schizophrenia. Biol Psychiatry 20:1015–1018
Schwartz BD, Winstead DK, Adinoff B (1983) Temporal integration deficit in visual information processing by chronic schizophrenics. Biol Psychiatry 18:1311–1320
Shapley R (1990) Visual sensitivity and parallel retinocortical channels. Annu Rev Psychol 41:635–658
Shapley R, Victor JD (1978) The effect of contrast on the transfer properties of cat ganglion cells. J Physiol 285:275–298
Slaghuis WL (1998) Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J Abnorm Psychol 107:49–62
Slaghuis WL, Bakker VJ (1995) Forward and backward masking of contour by light in positive- and negative symptom schizophrenia. J Abnorm Psychol 104:41–54
Slaghuis WL, Bishop AM (2001) Luminance flicker sensitivity in positive- and negative-symptom schizophrenia. Exp Brain Res 138:88–99
Slaghuis WL, Curran C (1999) Spatial frequency masking in positive- and negative-symptom schizophrenia. J Abnorm Psychol 108, 42–50
Slaghuis WL, Thompson A (2003) The effect of peripheral visual motion on contrast sensitivity in positive- and negative-symptom schizophrenia. Neuropsychologia 41:968–980
Smith AT (1991) Limits of velocity perception. In: Kulikowski JJ, Walsh V, Murray IJ (eds) Vision and visual dysfunction vol 5. Limits of vision. Macmillan Press/CRC Press, Broca Ranton FL
Smith AT, Edgar GK (1994) Antagonistic comparison of temporal frequency filter outputs as a basis for speed perception. Vision Res 34:253–266
Straus JS, Bartko JJ, Carpenter WT (1981) New directions in diagnosis: the longitudinal process of schizophrenia. Am J Psychiatry 138:945–958
Switkes E, Bradley A, De Valois KK (1988) Contrast dependence and mechanisms of masking interactions among chromatic and luminance gratings. J Opt Soc Am 5:1149–1162
Tebartz van Elst L, Greenlee MW, Foley JM, Lucking CH (1997) Contrast detection, discrimination and adaptation in patients with Parkinson’s disease and multiple system dystrophy. Brain 120:2219–2228
Tovee MJ (1994) How fast is the speed of thought. Curr Biol 4:1125–1127
Turvey MT (1973) On peripheral and central processes in vision: inferences from an information processing analysis of masking with pattern stimuli. Psychol Rev 80:1–52
Venables PH (1964) Input dysfunction in schizophrenia. In: Maher B (ed) Advances in experimental personality research, vol 1. Academic Press, New York
Vidyasagar TR, Kulikowski JJ, Lipnicki DM, Dreher B (2002) Convergence of parvocellular and magnocellular information channels in the primary visual cortex of the macaque. Eur J Neurosci 16:945–961
Wagman AM, Heinrichs DW, Carpenter WT (1987) Deficit and non-deficit forms of schizophrenia. Psychiatry Res 22:319–330
Wechsler D (1981) Wechsler Adult Intelligence Scale—revised. The Psychological Corporation/Harcourt Brace Jovanovich, New York
Weiner RU, Opler LA, Kay SR, Merriam AE, Papouchis N (1990) Visual information processing in positive, mixed, and negative schizophrenic syndromes. J Nerv Ment Dis 178:616–626
Weistein N, Wong E (1986) Figure-ground organisation affects the early visual processing of visual information. In: Arbib MA, Hanson AR (eds) Vision, brain, and cooperative computation. MIT Press, Cambridge MA, pp 209–230
Weistein N, Maguire W, Brannen J (1992) M and P pathways and the perception of figure and ground. In: JR Brannen (ed) Applications of parallel processing in vision. North-Holland, Amsterdam, pp 137–166
Wetherill GB, Levitt H (1965) Sequential estimation of points on a psychometric function. Br J Math Stat Psychol 18:1–10
Woodhouse JM, Barlow HB (1982) Spatial and temporal resolution and analysis. In: Barlow HB, Mollon JD (eds) The senses. Cambridge University Press, London, pp 133–164
Acknowledgements
The research was funded by an Australian Research Council grant to the author. The author gratefully acknowledges the assistance of Ms. M. Fahey in data collection, and the generous assistance of staff at the Richmond Fellowship and the Department of Health of Tasmania, Division of Psychiatry. The author gratefully acknowledges the contribution made by three anonymous referees.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slaghuis, W.L. Spatio-temporal luminance contrast sensitivity and visual backward masking in schizophrenia. Exp Brain Res 156, 196–211 (2004). https://doi.org/10.1007/s00221-003-1771-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1771-3