Abstract
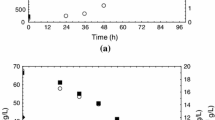
This work aimed at evaluating the total carotenoids production by a newly isolated Sporidiobolus pararoseus. Bioproduction was carried out in an orbital shaker, using 10% (w/v) of inoculum (25 °C, 180 rpm for 35 h), incubated for 120 h in a dark room. Liquid N2 and dimethylsulphoxide (DMSO) were used for cell rupture, and carotenoids were extracted with a solution of acetone/methanol (7:3, v/v). Optimization of carotenoids bioproduction was achieved by experimental design technique. Initially, a Plackett–Burman design was used for the screening of the most important factors, after the statistical analysis, a complete second-order design was carried out to optimize the concentration of total carotenoids in a conventional medium. Maximum concentration of 856 μg/L of total carotenoids was obtained in a medium containing 60 g/L of glucose, 15 g/L of peptone, and 15 g/L of malt extract, 25 °C, initial pH 4.0 and 180 rpm. Fermentation kinetics showed that the maximum concentration of total carotenoids was reached after 102 h of fermentation and that carotenoids bioproduction was associated with cell growth.




Similar content being viewed by others
References
Botella-Pavía P, Rodríguez-Concepción M (2006) Carotenoid biotechnology in plants for nutritionally improved foods. Physiol Plants 126:369–381
El-Agamey A, Lowe GM, Mcgarvey DJ, Mortensen A, Phillip DM, Truscott G, Young AJ (2004) Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys 430:37–48
Aksu Z, Eren AT (2005) Carotenoids production by the yeast Rhodotorula mucilaginosa: use of agricultural wastes as a carbon source. Process Biochem 40:2985–2991
Gu Z, Deming C, Yongbin H, Zhigang C, Feirong G (2008) Optimization of carotenoids extraction from Rhodobacter sphaeroides. Food Sci Technol 14:1082–1088
Aguilar CP, González M, Cifuentes AS, Silva M (2004) Growth and accumulation of total carotenoids in two strains of Dunaliella salina Teod. (Chlorophyceae) from the northern and central coast of Perú. J Chilean Chem Soc 49:69–74
Fazeli MR, Tofighi H, Samadi N, Jamalifar H (2006) Effects of salinity on b-carotene production by Dunaliella tertiolecta DCCBC 26 isolated from Urmia salt lake, North of Iran. Bioresour Technol 97:2453–2456
Po-Fung I, Feng C (2005) Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem 40:733–738
García-González M, Moreno J, Manzano JC, Florencio FJ, Guerrero MG (2005) Production of Dunaliella salina biomass rich in 9-cis-β-carotene and lutein in a closed tubular photobioreactor. J Biotechnol 115:81–90
Dufossé L, Galaup P, Yaron A, Arad SM, Blanc P, Murthy KNC (2005) Microorganisms and microalgae as sources of pigments for food use: a scientific oddity or an industrial reality. Trends Food Sci Technol 16:389–406
Johnson EA, Schroeder WA (1995) Microbial carotenoids. Adv Biochem Eng/Biotechnol 11:297–326
Goodwin TW (1980) The biochemistry of the carotenoids. Chapman & Hall, London
Hu ZC, Zheng YG, Wang Z, Shen YC (2006) pH control strategy in astaxanthin fermentation bioprocess by Xanthophyllomyces dendrorhous. Enzyme Microb Technol 39:586–590
Park PK, Kim EY, Chu KH (2007) Chemical disruption of yeast cells for the isolation of carotenoid pigments. Sep Pur Technol 53:148–152
Aksu Z, Eren AT (2007) Production of carotenoids by isolated yeast of Rhodotorula glutinis. Biochem Eng J 35:107–113
Tinoi J, Rakariyatham N, Deming RL (2005) Simplex optimization of carotenoid production by Rhodotorula glutinis using hydrolyzed mung bean waste flour as substrate. Process Biochem 40:2551–2557
Davoli P, Mierau V, Weber RWS (2004) Carotenoids and fatty acids in red yeasts Sporobolomyces roseus and Rhodotorula glutinis. Appl Biochem Microbiol 40(4):392–397
Razavi SH, March I (2006) Effect of temperature and pH on the growth kinetics and carotenoid production by Sporobolomyces ruberrimus H110 using technical glycerol as carbon source. Iranian J Chem Eng 23(3):59–64
Maldonade IR, Rodriguez-Amaya DB, Scamparini ARP (2008) Carotenoids of yeasts isolated from the Brazilian ecosystem. Food Chem 107:145–150
Valduga E, Valerio A, Treichel H, Di Luccio M, Furigo AJ (2008) Study of the bio-production of carotenoids by Sporidiobolus salmonicolor (CBS 2636) using pre-treated agro-industrial substrates. J Chem Technol Biotechnol 83:1267–1274
Valduga E, Valerio A, Treichel H, Furigo AJ, Di Luccio M (2009) Optimization of the bio-production of total carotenoids by Sporidiobolus salmonicolor (CBS 2636) using response surface technique. Food Bioproc Technol 2:415–421
Valduga E, Tatsch PO, Tiggemann L et al (2009) Evaluation of the conditions of carotenoids production in a synthetic medium by Sporidiobolus salmonicolor (CBS 2636) in a bioreactor. Int J Food Sci Technol 44:2445–2451
Valduga E, Tatsch PO, Tiggemann L, Treichel H, Toniazzo G, Zeni J, Di Luccio M, Furigo AJ (2009) Produção de carotenoides: microrganismos como fonte de pigmentos naturais. Quim Nova 32:2429–2436
Valduga E, Valerio A, Tatsch PO, Treichel H, Furigo AJ, Di Luccio M (2009) Assessment of cell disruption and carotenoids extraction from Sporidiobolus salmonicolor (CBS 2636). Food Bioproc Technol 2:234–238
Lim GB, Lee SY, Lee EK, Haam SJ, Kim WS (2002) Separation of astaxanthin from red yeast Phaffia rhodozyma by supercritical carbon dioxide extraction. Biochem Eng J 11:181–187
Liu YS, Wu JY, Ho KP (2006) Characterization of oxygen transfer conditions and their effects on Phaffia rhodozyma growth and carotenoid production in shake-flask cultures. Biochem Eng J 27:331–335
Valduga E, Schwartz CRM, Tatsch PO, Tiggemann L, Di Luccio M, Treichel H (2010) Evaluation of aeration and substrate concentration on the production of carotenoids by Sporidiobolus salmonicolor (CBS 2636) in bioreactor. Eur Food Res Technol 232:453–462
Zeni J, Colet R, Tiggemann L, Toniazzo G, Cansian RL, Di Luccio M, Oliveira D, Valduga E (2011) Screening of microorganisms for production of carotenoids. Cyta J Food, accepted for publication
Stirling D (2003) DNA extraction from fungi, yeast and bacteria. In: Bartlett JMS, Stirling D (eds) PCR protocols, 2nd edn. Humana Press, New York, p 226
Kurtzman CP (2006) Yeast species recognition from gene sequence analyses and other molecular methods. Mycoscience 47:65–71
Kurtzman CP, Robnett CJ (1998) Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large sub- unit 26S ribosomal DNA partial sequences. LWT 3:331–371
Fell JW, Boekhout T, Fonseca A, Scorzetti G, Statzell-Tallman A (2000) Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int J Syst Evolution Microbiol 3:1351–1371
Ewing B, Hillier L, Wendl MC (1998) Base-calling of automated sequencer traces using Phred.? I. accuracy? Assessment. Genome Res 8:175–185
Gordon D, Abajian C, Green P (1998) Consed: a graphical tool for sequence finishing. Genome Res 8:195–202
Bai FY, Zhao JH, Takashima M, Jia JH, Boekhout T, Nakase T (2002) Reclassification of the Sporobolomyces roseus and Sporidiobolus pararoseus complexes, wit the description of Sporobolomyces phaffii sp. nov. Int J Syst Evol Microbiol 52:2309–2314
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Saitou N, Nei M (1987) The neighbor-joining method: a newmethod for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Davies BH (1976) Chemistry and biochemistry of plant pigments. In: Goodwin TW (eds) Carotenoid. Academic Press, New York. pp 38–165
Silva C, Cabral JMS, Keulen FV (2004) Isolation of a β-total carotenoids over-producing soil bacterium, Sphingomonas sp. Biotechnol Let 26:257–262
Miller GL (1956) Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Buzzini P, Martini A, Gaetani M, Turchetti B, Pagnoni UM, Davoli P (2005) Optimization of carotenoid production by Rhodotorula graminis DBVPG 7021 as a function of trace element concentration by means of response surface analysis. Enzyme Microb Technol 36:687–692
Ramirez J, Gutierrrez H, Gschaedler A (2001) Optimization of astaxanthin production by Phaffia rhodozyma through factorial design and response surface methodology. J Biotechnol 88(3):259–268
Wang SL, Sun JS, Han BZ, Wu XZ (2007) Optimization of β-carotene production by Rhodotorula glutinis using high hydrostatic pressure and response surface methodology. J Food Sci 72:325–329
Buzzini P, Innocenti M, Turchetti B, Libkind D, van Broock M, Mulinacci N (2007) Carotenoid profiles of yeasts belonging to the genera Rhodotorula, Rhodosporidium, Sporobolomyces, and Sporidiobolus. Canadian J Microbiol 53:1024–1031
Choudari SM, Singhal R (2008) Media optimization for the production of β–carotene by Blakeslea trispora: a statistical approach. Biores Technol 99:722–730
Fang TJ, Wang JM (2002) Extractability of astaxanthin in a mixed culture of a carotenoid over-producing mutant of Xanthophyllomyces dendrourhous and Bacillus circulans in two-stage batch fermentation. Process Biochem 37:1235–1245
Liu YS, Wu JY (2007) Optimization of cell growth and carotenoid production of Xanthophyllomyces dendrorhous through statistical experiment design. Biochem Eng J 36:182–189
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cabral, M.M.S., Cence, K., Zeni, J. et al. Carotenoids production from a newly isolated Sporidiobolus pararoseus strain by submerged fermentation. Eur Food Res Technol 233, 159–166 (2011). https://doi.org/10.1007/s00217-011-1510-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-011-1510-0