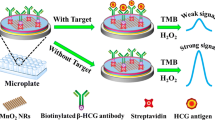
Abstract
A new near-infrared-based photothermal immunosensing strategy was developed for the sensitive and feasible detection of human chorionic gonadotropin (HCG) by use of a Prussian blue nanoparticle-based photothermal conversion system. Prussian blue nanospheres synthesized by the one-pot method were used for the labeling of anti-HCG detection antibody. A sandwich-type immunoreaction was initially conducted on a monoclonal anti-HCG antibody-coated microplate with a nanoparticle-labeled signal antibody. Accompanying formation of the sandwiched immunocomplex, Prussian blue nanospheres caused photothermal conversion under 980-nm laser irradiation, thereby resulting in an increase of the temperature of the detection system measured by a portable digital thermometer. The properties and factors influencing the analytical performance of the photothermal immunoassay were studied in detail. Under the optimal conditions, the Prussian blue nanoparticle-based photothermal immunoassay exhibited good temperature responses relative to target HCG concentrations within the dynamic range of 0.01–100 ng mL-1 at a low detection limit of 5.8 pg mL-1. This system also displayed good anti-interference behavior with regard to other cancer biomarkers, good reproducibility, and relatively long storage stability. The method accuracy was evaluated for analysis of human serum specimens, giving results that matched well with those obtained with a commercial HCG enzyme-linked immunosorbent assay kit. Importantly, this protocol is promising for advanced development of photothermal immunoassays.

Graphical abstract





Similar content being viewed by others
References
Wang W, Liu J, Zuo W. Immunotherapy in ovarian cancer. Surg Oncol Clin N Am. 2019;28:447–64.
Franier B, Thompson M. Early stage detection and screening of ovarian cancer: a research opportunity and significant challenge for biosensor technology. Biosens Bioelectron. 2019;135:71–81.
Fan J, Wang M, Wang C, Cao Y. Advances in human chorionic gonadotropin detection technologies: a review. Bioanalysis. 2017;9:1509–29.
Szabova L, Karim B, Gordon M, Lu L, Pate N, Ohler Z. A transplantable syngeneic allograft mouse model for nongestational chorocarcinoma of the ovary. Vet Pathol. 2019;56:399–403.
Zhong Y, Wang Y, Huang J, Xu X, Pan W, Gao S, et al. Association of hCG and LHCGR expression patterns with clinicopathological parameters in ovarian cancer. Pathol Res Pract. 2019;215:748–54.
Zhang W, Duan H, Chen R, Ma T, Zeng L, Leng Y, et al. Effect of different-sized gold nanoflowers on the detection performance of immunochromatographic assay from human chorionic gonadotropin detection. Talanta. 2019;194:604–10.
Camperi J, De Cock B, Pichon V, Combes A, Guibourdenche J, Fourier T, et al. First characterization by capillary electrophoresis of human chorionic gonadotropin at the intact level. Talanta. 2019;193:77–86.
Rizwan M, Hazmi M, Lim S, Ahmed M. A highly sensitive electrochemical detection of human chorionic gonadotropin on a carbon nano-onions/gold nanoparticles/polyethylene glycol nanocomposite modified glassy carbon electrode. J Electroanal Chem. 2019;833:462–70.
Egeland S, Reubsaet L, Paus E, Halvorsen T. Dual-immuno-MS technique for improved differentiation power in heterodimeric protein biomarker analysis: determination and differentiation of human chorionic gonadotropin variants in serum. Anal Bioanal Chem. 2016;408:7379–91.
Luo Z, Qi Q, Zhang L, Zeng R, Su L, Tang D. Branched polyethylenimine-modified upconversion nanohybrids-mediated photoelectrochemical immunoassay with synergistic effect of dual-purpose copper ions. Anal Chem. 2019;91:4149–56.
Yu Z, Tang Y, Cai G, Ren R, Tang D. Paper electrode-based flexible pressure sensor for point-of-care immunoassay with digital multimeter. Anal Chem. 2019;91:1222–6.
Lv S, Zhang K, Tang D. A new visual immunoassay for prostate-specific antigen using near-infrared excited CuxS nanocrystals and imaging on a smartphone. Analyst. 2019. https://doi.org/10.1039/c9an00724e.
Kimura H, Kitamori T, Sawada T. Critical increment of Lewis blood group antigen in serum by cancer found by photothermal immunoassay. Anal Biochem. 1999;274:98–103.
Li X, Yang L, Men C, Xie Y, Liu J, Zou H, et al. Photothermal soft nanoballs developed by loading plasmonic Cu2-xSe nanocrystals into liposomes for photothermal immunoassay of aflatoxin B1. Anal Chem. 2019;91:4444–50.
Liu Y, Wang W, Jiang Q, Wang F, Pang D, Liu X. Plasmonic and photothermal immunoassay via enzyme-triggered crystal growth on gold nanostars. Anal Chem. 2019;91:2086–92.
Fu G, Sanjay S, Zhou W, Brekken R, Kirken R, Li X. Exploration of nanoparticle-mediated photothermal effect of TMB-H2O2 colorimetric system and its application in a visual quantitative photothermal immunoassay. Anal Chem. 2018;90:5930–7.
Li J, Zhang F, Hu Z, Song W, Li G, Liang G, et al. Drug “pent-up” in hollow magnetic Prussian blue nanoparticles for NIR-induced chemo-photothermal tumor therapy with trimodal imaging. Adv Healthcare Mater. 2017;6:1700005.
Cai X, Gao W, Zhang L, Ma M, Liu T, Du W, et al. Enabling Prussian blue with tunable localized surface plasmon resonance: simultaneously enhanced dual-mode imaging and tumor photothermal therapy. ACS Nano. 2016;10:11115–26.
Cai S, Qian J, Yang S, Kuang L, Hua D. Acetylcysteine- decorated Prussian blue nanoparticles for strong photothermal sterilization and focal infection treatment. Colloids Surf B. 2019;181:31–8.
Fu G, Liu W, Feng S, Yue X. Prussian blue nanoparticles operate as a new generation of photothermal ablation agents for cancer therapy. Chem Commun. 2012;48:11567–9.
Cheng L, Gong H, Zhu W, Liu J, Wang X, Liu G, et al. PEGylated Prussian blue nanocubes as a theranostic agent for simultaneous cancer imaging and photothermal therapy. Biomaterials. 2014;35:9844–52.
Tang D, Tang J, Su B, Chen H, Huang J, Chen G. Highly sensitive electrochemical immunoassay for human IgG using double-encoded magnetic redox-active nanoparticles. Microchim Acta. 2010;171:457–64.
Cano-Mejia J, Bookstaver M, Sweeney E, Jewell C, Fernandes R. Prussian blue nanoparticle-based antigenicity and adjuvanticity trigger robust antitumor immune responses against neuroblastoma. Biomater Sci. 2019;7:1875–87.
Tang D, Su B, Tang J, Ren J, Chen G. Nanoparticle-based sandwich electrochemical immunoassay for carbohydrate antigen 125 with signal enhancement using enzyme-coated nanometer-sized enzyme-doped silica beads. Anal Chem. 2010;82:1527–34.
Zhang B, Liu B, Tang D, Niessner R, Chen G, Knopp D. DNA-based hybridization chain reaction for amplified bioelectronic signal and ultrasensitive detection of proteins. Anal Chem. 2012;84:5392–9.
Charoenkitamorn K, Trong Tue P, Chilae M, Chailapakul O, Takamura Y. Gold nanoparticle-labeled electrochemical immunoassay using open circuit potential for human chorionic gonadotropin detection. Electroanalysis. 2018;30:1766–72.
Partington L, Atkin S, Kilpatrick E, Morris S, Piper M, Lawrence N, et al. Electrochemical measurement of antibody-antigen recognition biophysics: Thermodynamic and kinetics of human chorionic gonadotropin (hCG) binding to redox-tagged antibodies. J Electroanal Chem. 2018;819:533–41.
Zhang A, Guo W, Ke H, Zhang X, Zhang H, Huang C, et al. Sandwich-format ECL immunosensor based on Au star@BSA-luminol nanocomposites for determination of human chorionic gonadotropin. Biosens Bioelectron. 2018;101:219–26.
Liang A, Li C, Li D, Luo Y, Wen G, Jiang Z. A facile and sensitive peptide-modulating graphene oxide nanoribbon catalytic nanoplasmon analytical platform for human chorionic gonadotropin. Int J Nanomed. 2017;12:8725–34.
Chiu N, Kuo C, Lin T, Chang C, Chen C. Ultra-high sensitivity of the non-immunological affinity of graphene oxide-peptide-based surface plasmon resonance biosensors to detection human chorionic gonadotropin. Biosens Bioelectron. 2017;94:351–7.
Cao L, Fang C, Zeng R, Zhao X, Jiang Y, Chen Z. Paper-based microfluidic devices for electrochemical immunofiltration analysis of human chorionic gonadotropin. Biosens Bioelectron. 2017;92:87–94.
Valipour A, Roushani M. Immunoassay for human chorionic gonadotropin based on glassy carbon electrode modified with an epitaxial nanocomposite. Anal Bioanal Chem. 2017;4:79–90.
Valipour A, Roushani M. Fabrication of an electrochemical immunosensor for determination of human chorionic gonadotropin based on PtNPs/cycteamine/AgNPs as an efficient interface. Anal Bioanal Chem Res. 2017;4:342–52.
Funding
We sincerely acknowledge the financial support of the National Natural Science Foundation of China (81772287 and 81371902), the Joint Project of Major Diseases in Xiamen City of China (3502Z20179044), and the Natural Science Foundation of Fujian Province, China (2016J01643).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
All procedures performed in studies involving human participants were approved by the First Affiliated Hospital of Xiamen University and Fujian Medical University and in accordance with the ethical standards of the First Affiliated Hospital of Xiamen University and Fujian Medical University ethics committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hong, G., Zhang, D., He, Y. et al. New photothermal immunoassay of human chorionic gonadotropin using Prussian blue nanoparticle-based photothermal conversion. Anal Bioanal Chem 411, 6837–6845 (2019). https://doi.org/10.1007/s00216-019-02049-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02049-w