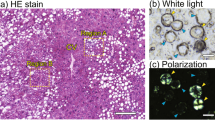
Abstract
Hepatic lipid accumulation, mainly in the form of triglycerides (TGs), is the hallmark of non-alcoholic fatty liver disease (NAFLD). To date, the spatial distribution of individual lipids in NAFLD-affected livers is not well characterized. This study aims to map the triglyceride distribution in normal human liver samples and livers with NAFLD and cirrhosis with imaging mass spectrometry (MALDI IMS). Specifically, whether individual triglyceride species differing by fatty acid chain length and degree of saturation correlate with the histopathological features of NAFLD as identified with classical H&E. Using a recently reported sodium-doped gold-assisted laser desorption/ionization IMS sample preparation, 20 human liver samples (five normal livers, five samples with simple steatosis, five samples with steatohepatitis, and five samples with cirrhosis) were analyzed at 10-μm lateral resolution. A total of 24 individual lipid species, primarily neutral lipids, were identified (22 TGs and two phospholipids). In samples with a low level of steatosis, TGs accumulated around the pericentral zone. In all samples, TGs with different degrees of side-chain saturation and side-chain length demonstrated differential distribution. Furthermore, hepatocytes containing macro lipid droplets were highly enriched in fully saturated triglycerides. This enrichment was also observed in areas of hepatocyte ballooning in samples with steatohepatitis and cirrhosis. In conclusion, macro lipid droplets in NAFLD are enriched in fully saturated triglycerides, indicating a possible increase in de novo lipogenesis that leads to steatohepatitis and cirrhosis.






Similar content being viewed by others
Abbreviations
- AuLDI IMS:
-
Gold-assisted laser desorption/ionization imaging mass spectrometry
- DAG:
-
Diglyceride
- DNL:
-
De novo lipogenesis
- FFAs:
-
Free fatty acids
- MALDI IMS:
-
Matrix-assisted laser desorption/ionization imaging mass spectrometry
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- OCT:
-
Optimal cutting temperature compound
- SFAs:
-
Saturated fatty acids
- SS:
-
Simple steatosis
- TG:
-
Triglycerides
References
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. https://doi.org/10.1002/hep.28431.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–23. https://doi.org/10.1002/hep.25762.
Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–55. https://doi.org/10.1053/j.gastro.2014.11.039.
McCormack L, Dutkowski P, El-Badry AM, Clavien P-A. Liver transplantation using fatty livers: always feasible? J Hepatol. 2011;54(5):1055–62. https://doi.org/10.1016/j.jhep.2010.11.004.
Hijmans BS, Grefhorst A, Oosterveer MH, Groen AK. Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Biochimie. 2014;96:121–9. https://doi.org/10.1016/j.biochi.2013.06.007.
Schleicher J, Tokarski C, Marbach E, Matz-Soja M, Zellmer S, Gebhardt R, et al. Zonation of hepatic fatty acid metabolism - the diversity of its regulation and the benefit of modeling. Biochim Biophys Acta. 2015;1851(5):641–56. https://doi.org/10.1016/j.bbalip.2015.02.004.
Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–51. https://doi.org/10.1172/JCI23621.
Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52(2):774–88. https://doi.org/10.1002/hep.23719.
de Vries JE, Vork MM, Roemen TH, de Jong YF, Cleutjens JP, van der Vusse GJ, et al. Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res. 1997;38(7):1384–94.
Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50(1):69–76.
Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr, Ory DS, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100(6):3077–82. https://doi.org/10.1073/pnas.0630588100.
Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147(2):943–51. https://doi.org/10.1210/en.2005-0570.
Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 2001;50(8):1771–7. https://doi.org/10.2337/diabetes.50.8.1771.
Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726–35. https://doi.org/10.1053/j.gastro.2013.11.049.
Debois D, Bralet MP, Le Naour F, Brunelle A, Laprevote O. In situ lipidomic analysis of nonalcoholic fatty liver by cluster TOF-SIMS imaging. Anal Chem. 2009;81(8):2823–31. https://doi.org/10.1021/ac900045m.
Wattacheril J, Seeley EH, Angel P, Chen H, Bowen BP, Lanciault C, et al. Differential intrahepatic phospholipid zonation in simple steatosis and nonalcoholic steatohepatitis. PLoS One. 2013;8(2):e57165. https://doi.org/10.1371/journal.pone.0057165.
Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–90. https://doi.org/10.1002/hep.21763.
Hall Z, Bond NJ, Ashmore T, Sanders F, Ament Z, Wang X, et al. Lipid zonation and phospholipid remodeling in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1165–80. https://doi.org/10.1002/hep.28953.
Hall Z, Chu Y, Griffin JL. Liquid extraction surface analysis mass spectrometry method for identifying the presence and severity of nonalcoholic fatty liver disease. Anal Chem. 2017;89(9):5161–70. https://doi.org/10.1021/acs.analchem.7b01097.
Norris JL, Caprioli RM. Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem Rev. 2013;113(4):2309–42. https://doi.org/10.1021/cr3004295.
Chaurand P. Imaging mass spectrometry of thin tissue sections: a decade of collective efforts. J Proteome. 2012;75(16):4883–92. https://doi.org/10.1016/j.jprot.2012.04.005.
McDonnell LA, Heeren RM. Imaging mass spectrometry. Mass Spectrom Rev. 2007;26(4):606–43. https://doi.org/10.1002/mas.20124.
Scupakova K, Soons Z, Ertaylan G, Pierzchalski KA, Eijkel GB, Ellis SR, et al. Spatial systems lipidomics reveals nonalcoholic fatty liver disease heterogeneity. Anal Chem. 2018. https://doi.org/10.1021/acs.analchem.7b05215.
Nishikawa K, Hashimoto M, Itoh Y, Hiroi S, Kusai A, Hirata F, et al. Detection of changes in the structure and distribution map of triacylglycerol in fatty liver model by MALDI-SpiralTOF. FEBS Open Bio. 2014;4:179–84. https://doi.org/10.1016/j.fob.2014.02.005.
Dufresne M, Masson JF, Chaurand P. Sodium-doped gold-assisted laser desorption ionization for enhanced imaging mass spectrometry of triacylglycerols from thin tissue sections. Anal Chem. 2016;88(11):6018–25. https://doi.org/10.1021/acs.analchem.6b01141.
Hamilton LK, Dufresne M, Joppe SE, Petryszyn S, Aumont A, Calon F, et al. Aberrant lipid metabolism in the forebrain niche suppresses adult neural stem cell proliferation in an animal model of Alzheimer’s disease. Cell Stem Cell. 2015;17(4):397–411. https://doi.org/10.1016/j.stem.2015.08.001.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. https://doi.org/10.1002/hep.20701.
Bemis KD, Harry A, Eberlin LS, Ferreira C, van de Ven SM, Mallick P, et al. Cardinal: an R package for statistical analysis of mass spectrometry-based imaging experiments. Bioinformatics. 2015;31(14):2418–20. https://doi.org/10.1093/bioinformatics/btv146.
Alexandrov T, Kobarg JH. Efficient spatial segmentation of large imaging mass spectrometry datasets with spatially aware clustering. Bioinformatics. 2011;27(13):i230–8. https://doi.org/10.1093/bioinformatics/btr246.
Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A. 2002;99(10):6567–72. https://doi.org/10.1073/pnas.082099299.
Chalasani N, Wilson L, Kleiner DE, Cummings OW, Brunt EM, Unalp A, et al. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J Hepatol. 2008;48(5):829–34. https://doi.org/10.1016/j.jhep.2008.01.016.
McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien P-A. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007;245(6):923–30. https://doi.org/10.1097/01.sla.0000251747.80025.b7.
Chu MJ, Dare AJ, Phillips AR, Bartlett AS. Donor hepatic steatosis and outcome after liver transplantation: a systematic review. J Gastrointest Surg. 2015;19(9):1713–24. https://doi.org/10.1007/s11605-015-2832-1.
Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59(5):1094–106. https://doi.org/10.1016/j.jhep.2013.06.017.
Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res. 2013;52(1):165–74. https://doi.org/10.1016/j.plipres.2012.10.004.
Luukkonen PK, Zhou Y, Sädevirta S, Leivonen M, Arola J, Orešič M, et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;64(5):1167–75. https://doi.org/10.1016/j.jhep.2016.01.002.
Acknowledgments
We would like to acknowledge the support of Stephanie Petrillo and Abdellatif Amri for technical support, the MUHC Liver Disease Biobank for all human specimens, and the patients who consented to providing samples to the Biobank, with whose support of this study would not have been possible.
Funding
This publication was supported by National Cancer Institute of the National Institutes of Health under award number R01CA198103 and by the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Informed consent was obtained from all patients through the McGill University Health Center Liver Disease Biobank. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the research ethics board of the McGill University Health Centre.
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 992 kb)
Rights and permissions
About this article
Cite this article
Alamri, H., Patterson, N.H., Yang, E. et al. Mapping the triglyceride distribution in NAFLD human liver by MALDI imaging mass spectrometry reveals molecular differences in micro and macro steatosis. Anal Bioanal Chem 411, 885–894 (2019). https://doi.org/10.1007/s00216-018-1506-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1506-8