Abstract
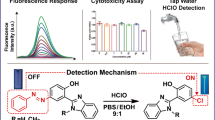
A simple Schiff base was prepared by mild condensation reaction between a coumarin fluorescent group and diaminomaleonitrile, and it could serve as an excellent fluorescent probe for fast detection of ClO− with high selectivity and sensitivity. Along with addition of ClO−, this probe fleetingly showed noteworthy “turn-on” phenomenon accompanied by an increase of fluorescence intensity and the change of emission color from yellow to blue. This change of fluorescence is so significant that it can be observed by the naked-eye under a handheld ultraviolet light of 365 nm. However, other common reactive oxygen species exhibited no or very little fluorescent response under the same conditions. The limit of detection of this probe toward ClO− had a sensitivity feature as low as 9.6 nM. On account of these excellent features of short response time, remarkable fluorescence and color signal changes, high sensitivity and selectivity, this probe was effectively used for the fluorescence detection of ClO− in water samples. The values of the relative standard deviation were between 1.41% and 2.91%. More importantly, this probe displays excellent imaging capability in cytoplasm as well as very low cell toxicity and was unambiguously applied to image ClO− in living cells.

A fluorescent probe based on coumarin for detection of ClO- was successfully developed, which can besuccessfully used for the detection of ClO− in living cells and in water sample because of the excellentfeatures including short response time, remarkable fluorescence and color signal changes, high sensitivityand selectivity











Similar content being viewed by others
References
Roos D, Winterbourn CC. Immunology – Lethal weapons. Science. 2002;296(5568):669–71. https://doi.org/10.1126/science.1071271.
Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281(52):39860–9. https://doi.org/10.1074/jbc.M605898200.
Pattison DI, Davies MJ. Evidence for rapid inter- and intramolecular chlorine transfer reactions of histamine and carnosine chloramines: implications for the prevention of hypochlorous-acid-mediated damage. Biochemistry USA. 2006;45(26):8152–62. https://doi.org/10.1021/bi060348s.
Bloomfield SF, Uso EE. The antibacterial properties of sodium hypochlorite and sodium dichloroisocyanurate as hospital disinfectants. J Hospital Infect. 1985;6(1):20–30. https://doi.org/10.1016/s0195-6701(85)80014-1.
Khatib S, Musa R, Vaya J. An exogenous marker: a novel approach for the characterization of oxidative stress. Bioorg Med Chem. 2007;15(11):3661–6. https://doi.org/10.1016/j.bmc.2007.03.052.
Yap YW, Whiteman M, Cheung NS. Chlorinative stress: an under appreciated mediator of neurodegeneration? Cell Signal. 2007;19(2):219–28. https://doi.org/10.1016/j.cellsig.2006.06.013.
Sam CH, Lu HK. The role of hypochlorous acid as one of the reactive oxygen species in periodontal disease. J Dent Sci. 2009;4(2):45–54. https://doi.org/10.1016/S1991-7902(09)60008-8.
Steinbeck MJ, Nesti LJ, Sharkey PF, Parvizi J. Myeloperoxidase and chlorinated-peptides in osteoarthritis: potential biomarkers of the disease. J Bone Miner Res. 2006;21:S144.
Maruyama Y, Lindholm B, Stenvinkel P. Inflammation and oxidative stress in ESRD – the role of myeloperoxidase. J Nephrol. 2004;17:S72–6.
Malle E, Buch T, Grone HJ. Myeloperoxidase in kidney disease. Kidney Int. 2003;64(6):1956–67. https://doi.org/10.1046/j.1523-1755.2003.00336.x.
Adam LC, Gordon G. Direct and sequential potentiometric determination of hypochlorite, chlorite, and chlorate ions when hypochlorite ion is present in large excess. Anal Chem. 1995;67(3):535–5340. https://doi.org/10.1021/Ac00099a009.
Thiagarajan S, Wu ZY, Chen SM. Amperometric determination of sodium hypochlorite at poly MnTAPP-nano Au film modified electrode. J Electroanal Chem. 2011;661(2):322–8. https://doi.org/10.1016/j.jelechem.2011.08.009.
Quentel F, Elleouet C, Madec CL. Determination of trace levels of chlorine dioxide in drinking water by electrochemistry. Analusis. 1996;24(5):199–203.
Shi LL, Li X, Zhou M, Muhammad F, Ding YB, Wei H. An arylboronate locked fluorescent probe for hypochlorite. Analyst. 2017;142(12):2104–8. https://doi.org/10.1039/c7an00467b.
Dehghani MH, Sanaei D, Ali I, Bhatnagar A. Removal of chromium(VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: kinetic modeling and isotherm studies. J Mol Liq. 2016;215:671–9. https://doi.org/10.1016/j.molliq.2015.12.057.
Karimi-Maleh H, Tahernejad-Javazmi F, Atar N, Lutfi M, Gupta VK, Ensafi AA. A novel DNA biosensor based on a pencil graphite electrode modified with polypyrrole/functionalized multiwalled carbon nanotubes for determination of 6-mercaptopurine anticancer drug. Ind Eng Chem Res. 2015;54(14):3634–9. https://doi.org/10.1021/ie504438z.
Yola ML, Gupta VK, Eren T, Sen AE, Atar N. A novel electro analytical nanosensor based on graphene oxide/silver nanoparticles for simultaneous determination of quercetin and morin. Electrochim Acta. 2014;120:204–11. https://doi.org/10.1016/j.electacta.2013.12.086.
Gupta VK, Kumar S, Singh R, Singh LP, Shoora SK, Sethi. Cadmium (II) ion sensing through p-tert-butyl calix[6]arene based potentiometric sensor. J Mol Liq. 2014;195:65–8. https://doi.org/10.1016/j.molliq.2014.02.001.
Gupta VK, Sethi B, Sharma RA, Agarwal S, Bharti A. Mercury selective potentiometric sensor based on low rim functionalized thiacalix [4]-arene as a cationic receptor. J Mol Liq. 2013;177:114–8. https://doi.org/10.1016/j.molliq.2012.10.008.
Karthikeyan S, Gupta VK, Boopathy R, Titus A, Sekaran G. A new approach for the degradation of high concentration of aromatic amine by heterocatalytic Fenton oxidation: kinetic and spectroscopic studies. J Mol Liq. 2012;173:153–63. https://doi.org/10.1016/j.molliq.2012.06.022.
Gupta VK, Nayak A, Agarwal S, Singhal B. Recent advances on potentiometric membrane sensors for pharmaceutical analysis. Comb Chem High T Scr. 2011;14(4):284–302.
Gupta VK, Ganjali MR, Norouzi P, Khani H, Nayak A, Agarwal S. Electrochemical analysis of some toxic metals by ion-selective electrodes. Crit Rev Anal Chem. 2011;41(4):282–313. https://doi.org/10.1080/10408347.2011.589773.
Yan LQ, Cui MF, Zhou Y, Ma Y, Qi ZJ. A simplified and turn-on fluorescence chemosensor based on coumarin derivative for detection of aluminium(III) ion in pure aqueous solution. Anal Sci. 2015;31(10):1055–9. https://doi.org/10.2116/analsci.31.1055.
Chen XQ, Wang F, Hyun JY, Wei TW, Qiang J, Ren XT, et al. Recent progress in the development of fluorescent, luminescent, and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem Soc Rev. 2016;45(10):2976–3016. https://doi.org/10.1039/c6cs00192k.
Liu W, Dong H, Zhang LM, Tian Y. Development of an efficient biosensor for the in vivo monitoring of Cu+ and pH in the brain: rational design and synthesis of recognition molecules. Angew Chem Int Ed. 2017;56(51):16328–32. https://doi.org/10.1002/anie.201710863.
Gupta VK, Mergu N, Kumawat LK, Singh AK. A reversible fluorescence "off-on-off" sensor for sequential detection of aluminum and acetate/fluoride ions. Talanta. 2015;144:80–9. https://doi.org/10.1016/j.talanta.2015.05.053.
Gupta VK, Karimi-Maleh H, Sadegh R. Simultaneous determination of hydroxylamine, phenol, and sulfite in water and waste water samples using a voltammetric nanosensor. Int J Electrochem Sci. 2015;10(1):303–16.
Gupta VK, Singh AK, Kumawat LK. Thiazole Schiff base turn-on fluorescent chemosensor for Al3+ ion. Sensor Actuat B–Chem. 2014;195:98–108. https://doi.org/10.1016/j.snb.2013.12.092.
Gupta VK, Singh LP, Singh R, Upadhyay N, Kaur SP, Sethi B. A novel copper (II) selective sensor based on Dimethyl 4,4' (o-phenylene) bis(3-thioallophanate) in PVC matrix. J Mol Liq. 2012;174:11–6. https://doi.org/10.1016/j.molliq.2012.07.016.
Srivastava SK, Gupta VK, Jain S. PVC-based 2,2,2-cryptand sensor for zinc ions. Anal Chem. 1996;68(7):1272–5. https://doi.org/10.1021/Ac9507000.
Srivastava SK, Gupta VK, Jain S. Determination of lead using a poly(vinyl chloride)-based crown-ether membrane. Analyst. 1995;120(2):495–8. https://doi.org/10.1039/An9952000495.
Huang X, Li ZP, Cao TT, Cai Q, Zeng CC, Fu H, et al. A methylene blue-based near-infrared fluorescent probe for rapid detection of hypochlorite in tap water and living cells. RSC Adv. 2018;8(26):14603–8. https://doi.org/10.1039/c8ra01037d.
Ji R, Qin K, Liu A, Zhu Y, Ge Y. A simple and fast-response fluorescent probe for hypochlorite in living cells. Tetrahedron Lett. 2018;59(24):2372–5. https://doi.org/10.1016/j.tetlet.2018.05.027.
Ding SS, Zhang Q, Xue SH, Feng GQ. Real-time detection of hypochlorite in tap water and biological samples by a colorimetric, ratiometric, and near-infrared fluorescent turn-on probe. Analyst. 2015;140(13):4687–93. https://doi.org/10.1039/c5an00465a.
Gong HL, Jiang Y, Hou RC, Ding XQ. A sensitive and selective fluorescent coumarin-based probe for detection of hypochlorite ion and its application to cellular imaging. J Fluoresc. 2016;26(2):403–6. https://doi.org/10.1007/s10895-015-1726-7.
Gong YQ, Yu B, Yang W, Zhang XL. Phosphorus and nitrogen co-doped carbon dots as a fluorescent probe for real-time measurement of reactive oxygen and nitrogen species inside macrophages. Biosens Bioelectron. 2016;79:822–8. https://doi.org/10.1016/j.bios.2016.01.022.
Tong HJ, Zhang YJ, Ma SN, Zhang MH, Wang N, Wang R, et al. A pinacol boronate caged NIAD-4 derivative as a near-infrared fluorescent probe for fast and selective detection of hypochlorous acid. Chinese Chem Lett. 2018;29(1):139–42. https://doi.org/10.1016/j.cclet.2017.07.007.
Lou XD, Zhang Y, Qin JG, Li Z. Colorimetric hypochlorite detection using an azobenzene acid in pure aqueous solutions and real application in tap water. Sensor Actuat B–Chem. 2012;161(1):229–34. https://doi.org/10.1016/j.snb.2011.10.024.
Wang KN, Sun PZ, Chao XJ, Cao DX, Mao ZW, Liu ZQ. A coumarin Schiff's base two-photon fluorescent probe for hypochlorite in living cells and zebrafish. RSC Adv. 2018;8(13):6904–9. https://doi.org/10.1039/c8ra00093j.
Xiong KM, Huo FJ, Zhang YB, Wen Y, Chao JB, Yin CX. A novel recognition mechanism based on aldehyde group oxidized into carboxyl group by hypochlorite for the materials of fluorescent probes. Sensor Actuat B–Chem. 2018;255:2378–83. https://doi.org/10.1016/j.snb.2017.09.042.
Yan LQ, Ma Y, Cui MF, Qi ZJ. A novel coumarin-based fluorescence chemosensor containing L-histidine for aluminium(III) ions in aqueous solution. Anal Methods–Uk. 2015;7(15):6133–8. https://doi.org/10.1039/c5ay01466b.
Yan LQ, Kong ZN, Xia Y, Qi ZJ. A novel coumarin-based red fluorogen with AIE, self-assembly, and TADF properties. New J Chem. 2016;40(8):7061–7. https://doi.org/10.1039/c6nj01296e.
Yan LQ, Kong ZN, Shen W, Du WQ, Zhou Y, Qi ZJ. An aggregation-induced emission (AIE) ratiometric fluorescent cysteine probe with an exceptionally large blue shift. RSC Adv. 2016;6(7):5636–40. https://doi.org/10.1039/c5ra22245a.
Zhang Z, Bai ZW, Ling Y, He LQ, Huang P, Gu HX, et al. Design, synthesis, and biological evaluation of novel furoxan-based coumarin derivatives as antitumor agents. Med Chem Res. 2018;27(4):1198–205. https://doi.org/10.1007/s00044-018-2140-x.
Gupta VK, Mergu N, Kumawat LK, Singh AK. Selective naked-eye detection of Magnesium (II) ions using a coumarin-derived fluorescent probe. Sensor Actuat B–Chem. 2015;207:216–23. https://doi.org/10.1016/j.snb.2014.10.044.
Ma Z, Sun W, Chen LZ, Li J, Liu ZZ, Bai HX, et al. A novel hydrazino-substituted naphthalimide-based fluorogenic probe for tert-butoxy radicals. Chem Commun. 2013;49(56):6295–7. https://doi.org/10.1039/c3cc42052c.
Li XH, Zhang GX, Ma HM, Zhang DQ, Li J, Zhu DB. 4,5-Dimethylthio-4'-[2-(9-anthryloxy)ethylthio]tetrathiafulvalene, a highly selective and sensitive chemiluminescence probe for singlet oxygen. J Am Chem Soc. 2004;126(37):11543–8. https://doi.org/10.1021/ja0481530.
Koide Y, Urano Y, Hanaoka K, Terai T, Nagano T. Development of an Si-rhodamine-based far-red to near-infrared fluorescence probe selective for hypochlorous acid and its applications for biological imaging. J Am Chem Soc. 2011;133(15):5680–2. https://doi.org/10.1021/ja111470n.
Yan LQ, Li RJ, Shen W, Qi ZJ. Multiple-color AIE coumarin-based Schiff bases and potential application in yellow OLEDs. J Lumin. 2018;194:151–5. https://doi.org/10.1016/j.jlumin.2017.10.032.
Acknowledgements
This work was supported by Foundation of Guilin University of Technology (GUTQDJJ2017), the Natural Science Foundation of Guangxi Province of China (Project No.2015GXNSFFA139005), and High Level Innovation Teams of Guangxi Colleges and Universities and Outstanding Scholars Program (Guijiaoren [2014]49).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest, and did not include any tests on people or animals.
Electronic supplementary material
ESM 1
(PDF 292 kb)
Rights and permissions
About this article
Cite this article
Yan, L., Hu, C. & Li, J. A fluorescence turn-on probe for rapid monitoring of hypochlorite based on coumarin Schiff base. Anal Bioanal Chem 410, 7457–7464 (2018). https://doi.org/10.1007/s00216-018-1352-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1352-8