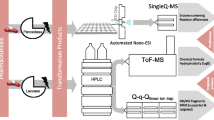
Abstract
Up to now, knowledge of enzymes capable of degrading various contaminants of emerging concern (CEC) is limited, which is especially due to the lack of rapid screening methods. Thus, a miniaturized high-throughput setup using a chip-based robotic nanoelectrospray ionization system coupled to mass spectrometry has been developed to rapidly screen enzymatic reactions with environmentally relevant CECs. Three laccases, two tyrosinases, and two peroxidases were studied for their ability to transform ten pharmaceuticals and benzotriazole. Acetaminophen was most susceptible to enzymatic conversion by horseradish peroxidase (HRP), laccase from Trametes versicolor (LccTV), and a tyrosinase from Agaricus bisporus (TyrAB). Diclofenac and mefenamic acid were converted by HRP and LccTV, whereas sotalol was solely amenable to HRP conversion. Benzotriazole, carbamazepine, gabapentin, metoprolol, primidone, sulfamethoxazole, and venlafaxine remained persistent in this study. The results obtained here emphasize that enzymes are highly selective catalysts and more effort is required in the use of fast monitoring technologies to find suitable enzyme systems. Despite the methodological limitations discussed in detail, the automated tool provides a routine on-line screening of various enzymatic reactions to identify potential enzymes that degrade CECs.

A chip-based robotic nano-ESI-MS tool to rapidly monitor enzymatic degradation of environmentally relevant emerging contaminants


Similar content being viewed by others
References
Petrović M, Gonzalez S, Barceló D. Analysis and removal of emerging contaminants in wastewater and drinking water. TrAC Trends Anal Chem. 2003;22(10):685–96. https://doi.org/10.1016/S0165-9936(03)01105-1.
Rao MA, Scelza R, Acevedo F, Diez MC, Gianfreda L. Enzymes as useful tools for environmental purposes. Chemosphere. 2014;107:145–62. https://doi.org/10.1016/j.chemosphere.2013.12.059.
Porter JL, Rusli RA, Ollis DL. Directed evolution of enzymes for industrial biocatalysis. Chembiochem. 2016;17(3):197–203. https://doi.org/10.1002/cbic.201500280.
Torres E, Bustos-Jaimes I, Le Borgne S. Potential use of oxidative enzymes for the detoxification of organic pollutants. Appl Catal B Environ. 2003;46(1):1–15. https://doi.org/10.1016/s0926-3373(03)00228-5.
Cooper V, Nicell J. Removal of phenols from a foundry wastewater using horseradish peroxidase. Water Res. 1996;30(4):954–64.
Nicell JA, Bewtra J, Taylor K, Biswas N, StPierre C. Enzyme catalyzed polymerization and precipitation of aromatic compounds from wastewater. Water Sci Technol. 1992;25(3):157–64.
Azevedo AM, Martins VC, Prazeres DMF, Vojinović V, Cabral JMS, Fonseca LP. Horseradish peroxidase: a valuable tool in biotechnology. Biotechnol Annu Rev. 2003;9:199–247. https://doi.org/10.1016/s1387-2656(03)09003-3.
van den Heuvel RH, Gato S, Versluis C, Gerbaux P, Kleanthous C, Heck AJ. Real-time monitoring of enzymatic DNA hydrolysis by electrospray ionization mass spectrometry. Nucleic Acids Res. 2005;33(10):e96–e.
Scheerle RK, Graßmann J, Letzel T. Enzymatic conversion continuously monitored with a robotic nanoESI-MS tool: experimental status. Anal Methods. 2011;3(4):822–30.
Grassmann J, Scheerle RK, Letzel T. Functional proteomics: application of mass spectrometry to the study of enzymology in complex mixtures. Anal Bioanal Chem. 2012;402(2):625–45. https://doi.org/10.1007/s00216-011-5236-4.
Burkhardt T, Kaufmann CM, Letzel T, Grassmann J. Enzymatic assays coupled with mass spectrometry with or without embedded liquid chromatography. Chembiochem. 2015;16(14):1985–92. https://doi.org/10.1002/cbic.201500325.
Stadlmair LF, Letzel T, Drewes JE, Graßmann J. Mass spectrometry based in vitro assay investigations on the transformation of pharmaceutical compounds by oxidative enzymes. Chemosphere. 2017;174:466–77.
Gasser CA, Yu L, Svojitka J, Wintgens T, Ammann EM, Shahgaldian P, et al. Advanced enzymatic elimination of phenolic contaminants in wastewater: a nano approach at field scale. Appl Microbiol Biotechnol. 2014;98(7):3305–16. https://doi.org/10.1007/s00253-013-5414-8.
Veitch NC. Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry. 2004;65(3):249–59. https://doi.org/10.1016/j.phytochem.2003.10.022.
Nguyen LN, Hai FI, Price WE, Leusch FD, Roddick F, Ngo HH, et al. The effects of mediator and granular activated carbon addition on degradation of trace organic contaminants by an enzymatic membrane reactor. Bioresour Technol. 2014;167:169–77. https://doi.org/10.1016/j.biortech.2014.05.125.
Ashe B, Nguyen LN, Hai FI, Lee D-J, van de Merwe JP, Leusch FDL, et al. Impacts of redox-mediator type on trace organic contaminants degradation by laccase: degradation efficiency, laccase stability and effluent toxicity. Int Biodeterior Biodegradation. 2016;113:169–76. https://doi.org/10.1016/j.ibiod.2016.04.027.
Schmidt A, Karas M, Dülcks T. Effect of different solution flow rates on analyte ion signals in nano-ESI MS, or: when does ESI turn into nano-ESI? J Am Soc Mass Spectrom. 2003;14(5):492–500. https://doi.org/10.1016/s1044-0305(03)00128-4.
Acknowledgements
The authors would like to thank AB Enzyme GmbH for the supply of two laccases and one tyrosinase. Furthermore, the authors thank Frank Porbeck (Advion BioSciences) for his assistance with the TriVersa NanoMate® system and various chips free of charge. The authors also gratefully thank the master’s students Rebecca Feind, Janine Storms, and Anastasia Vavelidou for their lab assistance and dedicated work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sector. We, the authors, declare that we have no competing interests. All authors are aware of and accept responsibility for this manuscript.
Electronic supplementary material
ESM 1
(PDF 150 kb)
Rights and permissions
About this article
Cite this article
Stadlmair, L.F., Letzel, T. & Graßmann, J. Monitoring enzymatic degradation of emerging contaminants using a chip-based robotic nano-ESI-MS tool. Anal Bioanal Chem 410, 27–32 (2018). https://doi.org/10.1007/s00216-017-0729-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0729-4