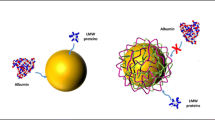
Abstract
Protein corona formed on nanomaterial surfaces play an important role in the bioavailability and cellular uptake of nanomaterials. Modification of surfaces with oligoethylene glycols (OEG) are a common way to improve the resistivity of nanomaterials to protein adsorption. Short-chain ethylene oxide (EO) oligomers have been shown to improve the protein resistance of planar Au surfaces. We describe the application of these EO oligomers for improved protein resistance of 30 nm spherical gold nanoparticles (AuNPs). Functionalized AuNPs were characterized using UV-Vis spectroscopy, dynamic light scattering (DLS), and zeta potential measurements. Capillary electrophoresis (CE) was used for separation and quantitation of AuNPs and AuNP-protein mixtures. Specifically, nonequilibrium capillary electrophoresis of equilibrium mixtures (NECEEM) was employed for the determination of equilibrium and rate constants for binding between citrate-stabilized AuNPs and two model proteins, lysozyme and fibrinogen. Semi-quantitative CE analysis was carried out for mixtures of EO-functionalized AuNPs and proteins, and results demonstrated a 2.5-fold to 10-fold increase in protein binding resistance to lysozyme depending on the AuNP surface functionalization and a 15-fold increase in protein binding resistance to fibrinogen for both EO oligomers examined in this study.

Using capillary electrophoresis, the addition of short-chained oligo(ethylene oxide) ligands to gold nanoparticles was shown to improve protein binding resistance up to 15-fold.





Similar content being viewed by others
Notes
Certain commercial equipment, instruments, and materials are identified in this paper to specify an experimental procedure as completely as possible. In no case does the identification of particular equipment or materials imply a recommendation or endorsement by the National Institute of Standards and Technology nor does it imply that the materials, instruments, or equipment are necessarily the best available for the purpose.
References
Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293–346. https://doi.org/10.1021/cr030698+.
Eustis S, el-Sayed MA. Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev. 2006;35(3):209–17. https://doi.org/10.1039/b514191e.
Han G, Ghosh P, Rotello VM. Functionalized gold nanoparticles for drug delivery. Nanomedicine (Lond). 2007;2(1):113–23. https://doi.org/10.2217/17435889.2.1.113.
Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine. Angew Chem Int Ed Engl. 2010;49(19):3280–94. https://doi.org/10.1002/anie.200904359.
Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, et al. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc Chem Res. 2008;41(12):1721–30. https://doi.org/10.1021/ar800035u.
Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41(12):1578–86. https://doi.org/10.1021/ar7002804.
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41(7):2740–79. https://doi.org/10.1039/C1CS15237H.
Klein J. Probing the interactions of proteins and nanoparticles. Proc Natl Acad Sci U S A. 2007;104(7):2029–30. https://doi.org/10.1073/pnas.0611610104.
Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A. 2008;105(38):14265–70. https://doi.org/10.1073/pnas.0805135105.
Lundqvist M, Stigler J, Cedervall T, Berggard T, Flanagan MB, Lynch I, et al. The evolution of the protein corona around nanoparticles: a test study. ACS Nano. 2011;5(9):7503–9. https://doi.org/10.1021/nn202458g.
Lynch I, Cedervall T, Lundqvist M, Cabaleiro-Lago C, Linse S, Dawson KA. The nanoparticle-protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv Colloid Interf Sci. 2007;134-135:167–74. https://doi.org/10.1016/j.cis.2007.04.021.
Treuel L, Nienhaus GU. Toward a molecular understanding of nanoparticle–protein interactions. Biophys Rev. 2012;4(2):137–47. https://doi.org/10.1007/s12551-012-0072-0.
Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Bombelli FB, et al. Physical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc. 2011;133(8):2525–34. https://doi.org/10.1021/ja107583h.
Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7(12):779–86. https://doi.org/10.1038/nnano.2012.207.
Fleischer CC, Payne CK. Nanoparticle-cell interactions: molecular structure of the protein corona and cellular outcomes. Acc Chem Res. 2014;47(8):2651–9. https://doi.org/10.1021/ar500190q.
Walkey CD, Olsen JB, Song F, Liu R, Guo H, Olsen DW, et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano. 2014;8(3):2439–55. https://doi.org/10.1021/nn406018q.
Treuel L, Brandholt S, Maffre P, Wiegele S, Shang L, Nienhaus GU. Impact of protein modification on the protein corona on nanoparticles and nanoparticle-cell interactions. ACS Nano. 2014;8(1):503–13. https://doi.org/10.1021/nn405019v.
Arvizo RR, Giri K, Moyano D, Miranda OR, Madden B, McCormick DJ, et al. Identifying new therapeutic targets via modulation of protein corona formation by engineered nanoparticles. PLoS One. 2012;7(3):e33650. https://doi.org/10.1371/journal.pone.0033650.
Zheng M, Davidson F, Huang X. Ethylene glycol monolayer protected nanoparticles for eliminating nonspecific binding with biological molecules. J Am Chem Soc. 2003;125(26):7790–1. https://doi.org/10.1021/ja0350278.
Zhang F, Skoda MW, Jacobs RM, Zorn S, Martin RA, Martin CM, et al. Gold nanoparticles decorated with oligo(ethylene glycol) thiols: protein resistance and colloidal stability. J Phys Chem A. 2007;111(49):12229–37. https://doi.org/10.1021/jp074293v.
Deng ZJ, Liang M, Toth I, Monteiro MJ, Minchin RF. Molecular interaction of poly(acrylic acid) gold nanoparticles with human fibrinogen. ACS Nano. 2012;6(10):8962–9. https://doi.org/10.1021/nn3029953.
Larson TA, Joshi PP, Sokolov K. Preventing protein adsorption and macrophage uptake of gold nanoparticles via a hydrophobic shield. ACS Nano. 2012;6(10):9182–90. https://doi.org/10.1021/nn3035155.
Boulos SP, Davis TA, Yang JA, Lohse SE, Alkilany AM, Holland LA, et al. Nanoparticle-protein interactions: a thermodynamic and kinetic study of the adsorption of bovine serum albumin to gold nanoparticle surfaces. Langmuir. 2013;29(48):14984–96. https://doi.org/10.1021/la402920f.
Murthy AK, Stover RJ, Hardin WG, Schramm R, Nie GD, Gourisankar S, et al. Charged gold nanoparticles with essentially zero serum protein adsorption in undiluted fetal bovine serum. J Am Chem Soc. 2013;135(21):7799–802. https://doi.org/10.1021/ja400701c.
Khan S, Gupta A, Verma NC, Nandi CK. Kinetics of protein adsorption on gold nanoparticle with variable protein structure and nanoparticle size. J Chem Phys. 2015;143(16):164709. https://doi.org/10.1063/1.4934605.
Pensa E, Cortes E, Corthey G, Carro P, Vericat C, Fonticelli MH, et al. The chemistry of the sulfur-gold interface: in search of a unified model. Acc Chem Res. 2012;45(8):1183–92. https://doi.org/10.1021/ar200260p.
Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, et al. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114(3):343–7. https://doi.org/10.1016/j.jconrel.2006.06.017.
Shenoy D, Fu W, Li J, Crasto C, Jones G, DiMarzio C, et al. Surface functionalization of gold nanoparticles using hetero-bifunctional poly(ethylene glycol) spacer for intracellular tracking and delivery. Int J Nanomedicine. 2006;1(1):51–7. https://doi.org/10.2147/nano.2006.1.1.51.
Pelaz B, del Pino P, Maffre P, Hartmann R, Gallego M, Rivera-Fernandez S, et al. Surface functionalization of nanoparticles with polyethylene glycol: effects on protein adsorption and cellular uptake. ACS Nano. 2015;9(7):6996–7008. https://doi.org/10.1021/acsnano.5b01326.
Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond). 2011;6(4):715–28. https://doi.org/10.2217/nnm.11.19.
Kenausis GL, Voros J, Elbert DL, Huang NP, Hofer R, Ruiz-Taylor L, et al. Poly(L-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: attachment mechanism and effects of polymer architecture on resistance to protein adsorption. J Phys Chem B. 2000;104(14):3298–309. https://doi.org/10.1021/jp993359m.
Zheng M, Li Z, Huang X. Ethylene glycol monolayer protected nanoparticles: synthesis, characterization, and interactions with biological molecules. Langmuir. 2004;20(10):4226–35. https://doi.org/10.1021/la035981i.
Zhang F, Dressen DG, Skoda MW, Jacobs RM, Zorn S, Martin RA, et al. Gold nanoparticles decorated with oligo(ethylene glycol) thiols: kinetics of colloid aggregation driven by depletion forces. Eur Biophys J. 2008;37(5):551–61. https://doi.org/10.1007/s00249-007-0255-y.
Schollbach M, Zhang F, Roosen-Runge F, Skoda MW, Jacobs RM, Schreiber F. Gold nanoparticles decorated with oligo(ethylene glycol) thiols: surface charges and interactions with proteins in solution. J Colloid Interface Sci. 2014;426:31–8. https://doi.org/10.1016/j.jcis.2014.03.052.
Vanderah DJ, Valincius G, Meuse CW. Self-assembled monolayers of methyl 1-thiahexa(ethylene oxide) for the inhibition of protein adsorption. Langmuir. 2002;18(12):4674–80. https://doi.org/10.1021/la025720t.
Vanderah DJ, La H, Naff J, Silin V, Rubinson KA. Control of protein adsorption: molecular level structural and spatial variables. J Am Chem Soc. 2004;126(42):13639–41. https://doi.org/10.1021/ja047744n.
Jeon SI, Andrade JD. Protein—surface interactions in the presence of polyethylene oxide. J Colloid Interface Sci. 1991;142(1):159–66. https://doi.org/10.1016/0021-9797(91)90044-9.
Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem Rev. 2005;105(4):1103–69. https://doi.org/10.1021/cr0300789.
Vanderah DJ, Vierling RJ, Walker ML. Oligo(ethylene oxide) self-assembled monolayers with self-limiting packing densities for the inhibition of nonspecific protein adsorption. Langmuir. 2009;25(9):5026–30. https://doi.org/10.1021/la803896a.
Vaish A, Vanderah DJ, Vierling R, Crawshaw F, Gallagher DT, Walker ML. Membrane protein resistance of oligo(ethylene oxide) self-assembled monolayers. Colloids Surf B Biointerfaces. 2014;122(0):552–8. https://doi.org/10.1016/j.colsurfb.2014.07.031.
Ostuni E, Grzybowski BA, Mrksich M, Roberts CS, Whitesides GM. Adsorption of proteins to hydrophobic sites on mixed self-assembled monolayers. Langmuir. 2003;19(5):1861–72. https://doi.org/10.1021/la020649c.
Zheng M, Huang X. Nanoparticles comprising a mixed monolayer for specific bindings with biomolecules. J Am Chem Soc. 2004;126(38):12047–54. https://doi.org/10.1021/ja047029d.
Patra A, Ding T, Engudar G, Wang Y, Dykas MM, Liedberg B, et al. Component-specific analysis of plasma protein corona formation on gold nanoparticles using multiplexed surface Plasmon resonance. Small. 2016;12(9):1174–82. https://doi.org/10.1002/smll.201501603.
Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci U S A. 2007;104(7):2050–5. https://doi.org/10.1073/pnas.0608582104.
Sasidharan A, Riviere JE, Monteiro-Riviere NA. Gold and silver nanoparticle interactions with human proteins: impact and implications in biocorona formation. J Mater Chem B. 2015;3(10):2075–82. https://doi.org/10.1039/c4tb01926a.
Treuel L, Malissek M, Grass S, Diendorf J, Mahl D, Meyer-Zaika W, et al. Quantifying the influence of polymer coatings on the serum albumin corona formation around silver and gold nanoparticles. J Nanopart Res. 2012;14(9):1102. https://doi.org/10.1007/s11051-012-1102-3.
Lacerda SH, Park JJ, Meuse C, Pristinski D, Becker ML, Karim A, et al. Interaction of gold nanoparticles with common human blood proteins. ACS Nano. 2010;4(1):365–79. https://doi.org/10.1021/nn9011187.
Bekdemir A, Stellacci F. A centrifugation-based physicochemical characterization method for the interaction between proteins and nanoparticles. Nat Commun. 2016;7:13121. https://doi.org/10.1038/ncomms13121.
Galievsky VA, Stasheuski AS, Krylov SN. Capillary electrophoresis for quantitative studies of biomolecular interactions. Anal Chem. 2015;87(1):157–71. https://doi.org/10.1021/ac504219r.
Ban E, Song EJ. Recent developments and applications of capillary electrophoresis with laser-induced fluorescence detection in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;929:180–6. https://doi.org/10.1016/j.jchromb.2013.04.028.
Righetti PG, Candiano G. Recent advances in electrophoretic techniques for the characterization of protein biomolecules: a poker of aces. J Chromatogr A. 2011;1218(49):8727–37. https://doi.org/10.1016/j.chroma.2011.04.011.
Guihen E. Nanoparticles in modern separation science. Trends Anal Chem. 2013;46:1–14. https://doi.org/10.1016/j.trac.2013.01.011.
Pyell U. Characterization of nanoparticles by capillary electromigration separation techniques. Electrophoresis. 2010;31(5):814–31. https://doi.org/10.1002/elps.200900555.
Surugau N, Urban PL. Electrophoretic methods for separation of nanoparticles. J Sep Sci. 2009;32(11):1889–906. https://doi.org/10.1002/jssc.200900071.
Aleksenko SS, Shmykov AY, Oszwaldowski S, Timerbaev AR. Interactions of tumour-targeting nanoparticles with proteins: potential of using capillary electrophoresis as a direct probe. Metallomics. 2012;4(11):1141–8. https://doi.org/10.1039/c2mt20141k.
Li N, Zeng S, He L, Zhong W. Probing nanoparticle--protein interaction by capillary electrophoresis. Anal Chem. 2010;82(17):7460–6. https://doi.org/10.1021/ac101627p.
Berezovski M, Krylov SN. Nonequilibrium capillary electrophoresis of equilibrium mixtures--a single experiment reveals equilibrium and kinetic parameters of protein-DNA interactions. J Am Chem Soc. 2002;124(46):13674–5. https://doi.org/10.1021/ja028212e.
Berezovski M, Nutiu R, Li Y, Krylov SN. Affinity analysis of a protein−aptamer complex using nonequilibrium capillary electrophoresis of equilibrium mixtures. Anal Chem. 2003;75(6):1382–6. https://doi.org/10.1021/ac026214b.
Krylov SN, Berezovski M. Non-equilibrium capillary electrophoresis of equilibrium mixtures - appreciation of kinetics in capillary electrophoresis. Analyst. 2003;128(6):571–5. https://doi.org/10.1039/b212913b.
Okhonin V, Krylova SM, Krylov SN. Nonequilibrium capillary electrophoresis of equilibrium mixtures, mathematical model. Anal Chem. 2004;76(5):1507–12. https://doi.org/10.1021/ac035259p.
Berezovski M, Drabovich A, Krylova SM, Musheev M, Okhonin V, Petrov A, et al. Nonequilibrium capillary electrophoresis of equilibrium mixtures: a universal tool for development of aptamers. J Am Chem Soc. 2005;127(9):3165–71. https://doi.org/10.1021/ja042394q.
Berezovski MV, Musheev MU, Drabovich AP, Jitkova JV, Krylov SN. Non-SELEX: selection of aptamers without intermediate amplification of candidate oligonucleotides. Nat Protoc. 2006;1(3):1359–69. https://doi.org/10.1038/nprot.2006.200.
Hackley VA, Clogston JD (2010) NIST - NCL Joint Assay Protocol, PCC-1 Version 1.1 Measuring the Size of Nanoparticles in Aqueous Media Using BatchMode Dynamic Light Scattering. https://ncl.cancer.gov/sites/default/files/protocols/NCL_Method_PCC-1.pdf.
Light TS, Kingman B, Bevilacqua AC (1995) The Conductivity of Low Concentrations of CO2 Dissolved in Ultrapure Water from 0–100°C. Paper presented at the 209th American Chemical Society National Meeting, Anaheim.
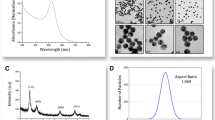
Haiss W, Thanh NT, Aveyard J, Fernig DG. Determination of size and concentration of gold nanoparticles from UV-vis spectra. Anal Chem. 2007;79(11):4215–21. https://doi.org/10.1021/ac0702084.
Egerton RF, Li P, Malac M. Radiation damage in the TEM and SEM. Micron. 2004;35(6):399–409. https://doi.org/10.1016/j.micron.2004.02.003.
Riley KR, Liu S, Yu G, Libby K, Cubicciotti R, Colyer CL. Using capillary electrophoresis to characterize polymeric particles. J Chromatogr A. 2016;1463:169–75. https://doi.org/10.1016/j.chroma.2016.08.017.
Qu H, Mudalige TK, Linder SW. Capillary electrophoresis/inductively-coupled plasma-mass spectrometry: development and optimization of a high resolution analytical tool for the size-based characterization of nanomaterials in dietary supplements. Anal Chem. 2014;86(23):11620–7. https://doi.org/10.1021/ac5025655.
Liu F-K, Wei G-T. Adding sodium dodecylsulfate to the running electrolyte enhances the separation of gold nanoparticles by capillary electrophoresis. Anal Chim Acta. 2004;510(1):77–83. https://doi.org/10.1016/j.aca.2003.12.064.
Acknowledgements
KRR and CMS acknowledge funding and support from the National Academy of Sciences - National Research Council Postdoctoral Research Associateship Program. ITW recognizes the support of the NIST Summer Undergraduate Research Fellowship (SURF) Program.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
M. L. Walker and D. J. Vanderah conceived the initial project; I. T. Wood and M. L. Walker performed initial studies; K.R. Riley and C.M. Sims refined the project and contributed equally to essential experimentation and composition of the completed work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any research with human or animal subjects.
Electronic supplementary material
ESM 1
(PDF 9.52 mb)
Rights and permissions
About this article
Cite this article
Riley, K.R., Sims, C.M., Wood, I.T. et al. Short-chained oligo(ethylene oxide)-functionalized gold nanoparticles: realization of significant protein resistance. Anal Bioanal Chem 410, 145–154 (2018). https://doi.org/10.1007/s00216-017-0704-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0704-0