Abstract
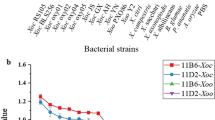
Multiarray on a test strip (MATS) was developed for the detection of eight important potato pathogens. The proposed assay combines the rapidity of immunochromatography with the high throughput of array techniques. The test zone of the immunochromatographic strip comprises ordered rows of spots containing antibodies specific for different potato pathogens. The assay benefits from the simplicity of immunochromatography; colored immune complexes form at the corresponding spots within the test zone. The presence and intensity of the coloration are used for identification of the target pathogens. The MATS was applied to the simultaneous detection of eight priority potato pathogens, characterized by the following limits of detection: 1 ng/mL for potato virus X and the ordinary type of potato virus Y, 10 ng/mL for potato virus M, 20 ng/mL for potato leaf roll virus, 40 ng/mL for necrotic-type potato virus Y, 100 ng/mL for potato virus S, 300 ng/mL for potato virus A, and 104 cells/mL for Clavibacter michiganensis subsp. sepedonicus. Analysis time was 15 min. The observed sensitivity of the MATS was comparable to the traditional enzyme-linked immunosorbent assay. The developed technique was tested on potato leaf extracts, and its efficiency for on-site control of the pathogens was confirmed in 100 % by commercial LFIA test strips.

Location of binding zones in the developed multiarray on a test strip (MATS) for simultaneous detection of eight pathogens





Similar content being viewed by others
References
Dzantiev BB, Byzova NA, Urusov AE, Zherdev AV. Immunochromatographic methods in food analysis. TrAC-Trend Anal Chem. 2014;55:81–93.
Marquette CA, Corgier BP, Blum LJ. Recent advances in multiplex immunoassays. Bioanalysis. 2012;4(8):927–36.
Tighe PJ, Ryder RR, Todd I, Fairclough LC. ELISA in the multiplex era: potentials and pitfalls. Proteom Clin Appl. 2015;9(3–4):406–22.
Charermroj R, Himananto O, Seepiban C, Kumpoosiri M, Warin N, Gajanandana O, et al. Antibody array in a multiwell plate format for the sensitive and multiplexed detection of important plant pathogens. Anal Chem. 2014;86(14):7049–56.
Ngom B, Guo YC, Wang XL, Bi DR. Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: a review. Anal Bioanal Chem. 2010;397(3):1113–35.
Posthuma-Trumpie G, Korf J, Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393(2):569–82.
Gantelius J, Bass T, Sjoberg R, Nilsson P, Andersson-Svahn H. A lateral flow protein microarray for rapid and sensitive antibody assays. Int J Mol Sci. 2011;12:7748–59.
Taranova NA, Byzova NA, Zaiko VV, Starovoitova TA, Vengerov YY, Zherdev AV, et al. Integration of lateral flow and microarray technologies for multiplex immunoassay: application to the determination of drugs of abuse. Microchim Acta. 2013;180(11–12):1165–72.
Tighe P, Negm O, Todd I, Fairclough L. Utility, reliability and reproducibility of immunoassay multiplex kits. Methods. 2013;61(1):23–9.
Gantelius J, Hamsten C, Neiman M, Schwenk JM, Persson A, Andersson-Svahn H. A lateral flow protein microarray for rapid determination of contagious bovine pleuropneumonia status in bovine serum. J Microbiol Methods. 2010;82(1):11–8.
Reutersward P, Gantelius J, Andersson Svahn H. An 8 minute colorimetric paper-based reverse phase vertical flow serum microarray for screening of hyper IgE syndrome. Analyst (Cambridge, U K). 2015;140:7327–34.
Chinnasamy T, Segerink LI, Nystrand M, Gantelius J, Svahn HA. A lateral flow paper microarray for rapid allergy point of care diagnostics. Analyst (Cambridge, U K). 2014;139:2348–54.
Narayanasamy P. Diagnosis of viral and viroid diseases of plants. In: Microbial plant pathogens-detection and disease diagnosis. Springer Netherlands; 2011. pp 295–312.
Narayanasamy P. Diagnosis of bacterial diseases of plants. In: Microbial plant pathogens-detection and disease diagnosis. Springer Netherlands; 2011. pp 233–246.
De Boer SH, Lopez MM. New grower-friendly methods for plant pathogen monitoring. Annu Rev Phytopathol. 2012;50:197–218.
Nezhad AS. Future of portable devices for plant pathogen diagnosis. Lab Chip. 2014;14(16):2887–904.
Samsatly J, Jawhari M, Najjar C, Sobh H, Abou-Jawdah Y. Modification of serological techniques and their evaluation for detection of potato viruses in seed certification related activities. Crop Prot. 2014;61:51–7.
Charlermroj R, Himananto O, Seepiban C, Kumpoosiri M, Warin N, Oplatowska M, et al. Multiplex detection of plant pathogens using a microsphere immunoassay technology. PLoS One. 2013;8(4):e62344. doi:10.1371/journal.pone.0062344.
Bergervoet JHW, Peters J, van Beckhoven J, van den Bovenkamp GW, Jacobson JW, van der Wolf JM. Multiplex microsphere immuno-detection of potato virus Y, X and PLRV. J Virol Methods. 2008;149(1):63–8.
Zhang M, Chen W, Chen X, Zhang Y, Lin X, Wu Z, et al. Multiplex immunoassays of plant viruses based on functionalized upconversion nanoparticles coupled with immunomagnetic separation. J Nanomater. 2013;2013:8. doi:10.1155/2013/317437.
Potrykus M, Sledz W, Golanowska M, Slawiak M, Binek A, Motyka A, et al. Simultaneous detection of major blackleg and soft rot bacterial pathogens in potato by multiplex polymerase chain reaction. Ann Appl Biol. 2014;165:474–87.
Ge BB, Li Q, Liu GJ, Lu MG, Li SF, Wang HQ. Simultaneous detection and identification of four viruses infecting pepino by multiplex RT-PCR. Arch Virol. 2013;158(6):1181–7.
Peiro A, Pallas V, Sanchez-Navarro JA. Simultaneous detection of eight viruses and two viroids affecting stone fruit trees by using a unique polyprobe. Eur J Plant Pathol. 2012;132(4):469–75.
Safenkova IV, Zaitsev IA, Pankratova GK, Varitsev YA, Zherdev AV, Dzantiev BB. Lateral flow immunoassay for rapid detection of potato ring rot caused by Clavibacter michiganensis subsp sepedonicus. Appl Biochem Microbiol. 2014;50(6):675–82.
Byzova NA, Safenkova IV, Chirkov SN, Zherdev AV, Blintsov AN, Dzantiev BB, et al. Development of immunochromatographic test systems for express detection of plant viruses. Appl Biochem Microbiol. 2009;45(2):204–9.
Safenkova IV, Zherdev AV, Dzantiev BB. Factors influencing the detection limit of the lateral-flow sandwich immunoassay: a case study with potato virus X. Anal Bioanal Chem. 2012;403(6):1595–605.
Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241(105):20–2.
Safenkova IV, Zherdev AV, Dzantiev BB. Correlation between the composition of multivalent antibody conjugates with colloidal gold nanoparticles and their affinity. J Immunol Methods. 2010;357(1−2):17–25.
Panferov VG, Safenkova IV, Varitsev YA, Drenova NV, Kornev KP, Zherdev AV, Dzantiev BB Development of the sensitive lateral flow immunoassay with silver enhancement for the detection of Ralstonia solanacearum in potato tubers. Talanta. 2016;152:521–30.
Byzova NA, Safenkova IV, Chirkov SN, Avdienko VG, Guseva AN, Mitrofanova IV, et al. Interaction of plum pox virus with specific colloidal gold-labeled antibodies and development of immunochromatographic assay of the virus. Biochemistry (Moscow). 2010;75(11):1393–403.
Zhang XQ, Li D, Wang C, Zhi X, Zhang CL, Wang K, et al. A CCD-based reader combined quantum dots-labeled lateral flow strips for ultrasensitive quantitative detection of anti-HBs antibody. J Biomed Nanotechnol. 2012;8(3):372–9.
Yan Z, Zhou L, Zhao Y, Wang J, Huang L, Hu K, et al. Rapid quantitative detection of Yersinia pestis by lateral-flow immunoassay and up-converting phosphor technology-based biosensor. Sens Actuators, B. 2006;119(2):656–63.
Acknowledgments
This study was supported by the Russian Science Foundation (Grant No. 14-16-00149).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection Immunoanalysis for Environmental Monitoring and Human Health with guest editors Shirley J. Gee, Ivan R. Kennedy, Alice Lee, Hideo Ohkawa, Tippawan Prapamontol, and Ting Xu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 732 kb)
Rights and permissions
About this article
Cite this article
Safenkova, I.V., Pankratova, G.K., Zaitsev, I.A. et al. Multiarray on a test strip (MATS): rapid multiplex immunodetection of priority potato pathogens. Anal Bioanal Chem 408, 6009–6017 (2016). https://doi.org/10.1007/s00216-016-9463-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9463-6