Abstract
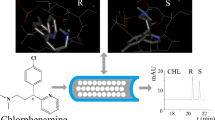
In recent years, chiral ionic liquids (CILs) have attracted more and more attention in the field of enantioseparation. In this study, two novel spirocyclic chiral ionic liquids, 1-butyl-3-methylimidazolium(T-4)-bis[(2S)-2-(hydroxy-κO)-3-methylbutanoato-κO]borate (BMIm+BLHvB−) and 1-butyl-3-methylimidazolium (T-4)-bis[(αS)-α-(hydroxy-κO)-4-methylbenzeneacetato-κO]borate (BMIm+BSMB−), were applied for the first time in capillary electrophoresis (CE) to establish synergistic systems for enantiomeric separation. Significantly improved separations of five tested analytes were observed in the CILs synergistic systems based on three β-cyclodextrin derivatives (CD), compared with conventional single CD separation systems. Several principal parameters such as CILs concentration, cyclodextrin concentration, buffer pH, and applied voltage were systematically investigated with BMIm+BLHvB−/hydroxypropyl-β-CD selected as a model system to optimize the enantioseparation. Molecular modeling was applied to further demonstrate the chiral recognition mechanism of the CILs/hydroxypropyl-β-CD synergistic system, which showed a good agreement with the experimental results.






Similar content being viewed by others
References
Wang S, Dai Y, Wu J, Zhou J, Tang J, Tang W. Methoxyethylammonium monosubstituted β-cyclodextrin as the chiral selector for enantioseparation in capillary electrophoresis. J Chromatogr A. 2013;1277:84–92.
Deñola NL, Quiming NS, Catabay AP, Saito Y, Jinno K. Optimization of capillary electrophoretic enantioseparation for basic drugs with native β-CD as a chiral selector. Electrophoresis. 2006;27(12):2367–75.
Nishi H. Enantioselectivity in chiral capillary electrophoresis with polysaccharides. J Chromatogr A. 1997;792:327–47.
Maier V, Ranc V, Švidrnoch M, Petr J, Ševčík J, Tesařová E, et al. Study on the use of boromycin as a chiral selector in capillary electrophoresis. J Chromatogr A. 2012;1237:128–32.
Haginaka J. Enantiomer separation of drugs by capillary electrophoresis using proteins as chiral selectors. J Chromatogr A. 2000;875(1):235–54.
Pandey S. Analytical applications of room-temperature ionic liquids: a review of recent efforts. Anal Chim Acta. 2006;556(1):38–45.
Ding J, Welton T, Armstrong DW. Chiral ionic liquids as stationary phases in gas chromatography. Anal Chem. 2004;76(22):6819–22.
Huang K, Zhang X, Armstrong DW. Ionic cyclodextrins in ionic liquid matrices as chiral stationary phases for gas chromatography. J Chromatogr A. 2010;1217(32):5261–73.
Marszałł MP, Kaliszan R. Application of ionic liquids in liquid chromatography. Crit Rev Anal Chem. 2007;37(2):127–40.
Li J, Han H, Wang Q, Liu X, Jiang S. Polymeric ionic liquid as a dynamic coating additive for separation of basic proteins by capillary electrophoresis. Anal Chim Acta. 2010;674(2):243–8.
Jin Y, Chen C, Meng L, Chen J, Li M, Zhu Z. Simultaneous and sensitive capillary electrophoretic enantioseparation of three β-blockers with the combination of achiral ionic liquid and dual CD derivatives. Talanta. 2012;89:149–54.
Vaher M, Koel M, Kazarjan J, Kaljurand M. Capillary electrophoretic analysis of neutral carbohydrates using ionic liquids as background electrolytes. Electrophoresis. 2011;32(9):1068–73.
François Y, Varenne A, Juillerat E, Servais A-C, Chiap P, Gareil P. Nonaqueous capillary electrophoretic behavior of 2-aryl propionic acids in the presence of an achiral ionic liquid: a chemometric approach. J Chromatogr A. 2007;1138(1):268–75.
Zhang Q, Du Y, Du S. Evaluation of ionic liquids-coated carbon nanotubes modified chiral separation system with chondroitin sulfate E as chiral selector in capillary electrophoresis. J Chromatogr A. 2014;1339:185–91.
François Y, Varenne A, Juillerat E, Villemin D, Gareil P. Evaluation of chiral ionic liquids as additives to cyclodextrins for enantiomeric separations by capillary electrophoresis. J Chromatogr A. 2007;1155(2):134–41.
Rousseau A, Florence X, Pirotte B, Varenne A, Gareil P, Villemin D, et al. Development and validation of a nonaqueous capillary electrophoretic method for the enantiomeric purity determination of a synthetic intermediate of new 3,4-dihydro-2,2-dimethyl-2H-1-benzopyrans using a single-isomer anionic cyclodextrin derivative and an ionic liquid. J Chromatogr A. 2010;1217(51):7949–55.
Wang B, He J, Bianchi V, Shamsi SA. Combined use of chiral ionic liquid and cyclodextrin for MEKC: part I. Simultaneous enantioseparation of anionic profens. Electrophoresis. 2009;30(16):2812–9.
Wang B, He J, Bianchi V, Shamsi SA. Combined use of chiral ionic liquid and CD for MEKC: part II. Determination of binding constants. Electrophoresis. 2009;30(16):2820–8.
Zhang J, Du Y, Zhang Q, Chen J, Xu G, Yu T, et al. Investigation of the synergistic effect with amino acid-derived chiral ionic liquids as additives for enantiomeric separation in capillary electrophoresis. J Chromatogr A. 2013;1316:119–26.
Zhang J, Du Y, Zhang Q, Lei Y. Evaluation of vancomycin-based synergistic system with amino acid ester chiral ionic liquids as additives for enantioseparation of non-steroidal anti-inflamatory drugs by capillary electrophoresis. Talanta. 2014;119:193–201.
Zhang Q, Du Y. Evaluation of the enantioselectivity of glycogen-based synergistic system with amino acid chiral ionic liquids as additives in capillary electrophoresis. J Chromatogr A. 2013;1306:97–103.
Xu G, Du Y, Du F, Chen J, Yu T, Zhang Q, et al. Establishment and evaluation of the novel tetramethylammonium-L-hydroxyproline chiral ionic liquid synergistic system based on clindamycin phosphate for enantioseparation by capillary electrophoresis. Chirality. 2015;27(9):598–604.
Ma Z, Zhang L, Lin L, Ji P, Guo X. Enantioseparation of rabeprazole and omeprazole by nonaqueous capillary electrophoresis with an ephedrine-based ionic liquid as the chiral selector. Biomed Chromatogr. 2010;24(12):1332–7.
Rizvi SAA, Shamsi SA. Synthesis, characterization, and application of chiral ionic liquids and their polymers in micellar electrokinetic chromatography. Anal Chem. 2006;78(19):7061–9.
Tran CD, Mejac I. Chiral ionic liquids for enantioseparation of pharmaceutical products by capillary electrophoresis. J Chromatogr A. 2008;1204(2):204–9.
Stavrou IJ, Kapnissi-Christodoulou CP. Use of chiral amino acid ester-based ionic liquids as chiral selectors in CE. Electrophoresis. 2013;34(4):524–30.
Liu R, Du Y, Chen J, Zhang Q, Du S, Feng Z. Investigation of the enantioselectivity of tetramethylammonium L-hydroxyproline ionic liquid as a novel chiral ligand in ligand-exchange CE and ligand-exchange MEKC. Chirality. 2015;27(1):58–63.
Zhang Q, Du Y, Du S, Zhang J, Feng Z, Zhang Y, et al. Tetramethylammonium- lactobionate: a novel ionic liquid chiral selector based on saccharides in capillary electrophoresis. Electrophoresis. 2015;36(9-10):1216–23.
Bednarek E, Bocian W, Michalska K. NMR and molecular modeling study, as complementary techniques to capillary electrophoresis method to elucidate the separation mechanism of linezolid enantiomers. J Chromatogr A. 2008;1193(1):164–71.
Li W, Tan G, Zhao L, Chen X, Zhang X, Zhu Z, et al. Computer-aided molecular modeling study of enantioseparation of iodiconazole and structurally related triadimenol analogues by capillary electrophoresis: chiral recognition mechanism and mathematical model for predicting chiral separation. Anal Chim Acta. 2012;718:138–47.
Yu S, Lindeman S, Tran CD. Chiral ionic liquids: synthesis, properties, and enantiomeric recognition. J Org Chem. 2008;73(7):2576–91.
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30(16):2785–91.
Sanner M, Huey R, Dallakyan S, Karnati S, Lindstrom W, Morris G, et al. (2007) AutoDockTools, version 1.4. 5. The Scripps Research Institute, La Jolla.
Ravichandran S, Collins JR, Singh N, Wainer IW. A molecular model of the enantioselective liquid chromatographic separation of (R, S)-ifosfamide and its N-dechloroethylated metabolites on a teicoplanin aglycon chiral stationary phase. J Chromatogr A. 2012;1269:218–25.
Wang HY, Wang JJ, Zhang SL, Pei YC, Zhuo KL. Ionic association of the ionic liquids [C4mim][BF4], [C4mim][PF6], and [Cnmim]Br in molecular solvents. ChemPhysChem. 2009;10(14):2516–23.
Yee P, Shah JK, Maginn EJ. State of hydrophobic and hydrophilic ionic liquids in aqueous solutions: are the ions fully dissociated? J Phys Chem B. 2013;117(41):12556–66.
Shekaari H, Mousavi SS. Conductometric studies of aqueous ionic liquids, 1-alkyl-3-methylimidazolium halide, solutions at T= 298.15-328.15 K. Fluid Phase Equilib. 2009;286(2):120–6.
Katsuta S, Ogawa R, Yamaguchi N, Ishitani T, Takeda Y. Ion pair formation of 1-alkyl-3-methylimidazolium salts in water. J Chem Eng Data. 2007;52(1):248–51.
Yan C, Xiu Z, Li X, Hao C. Molecular modeling study of β-cyclodextrin complexes with (+)-catechin and (-)-epicatechin. J Mol Graph Model. 2007;26(2):420–8.
Acknowledgments
This work was supported by the Project of National Natural Science Foundation of China (Nos. 81373378 and 81072610), the Natural Science Foundation of Jiangsu Province (Program No. BK20150697) and the Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, Y., Du, S., Feng, Z. et al. Evaluation of synergistic enantioseparation systems with chiral spirocyclic ionic liquids as additives by capillary electrophoresis. Anal Bioanal Chem 408, 2543–2555 (2016). https://doi.org/10.1007/s00216-016-9356-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9356-8