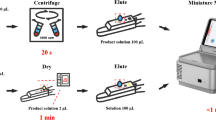
Abstract
Desorption electrospray ionization (DESI) mass spectrometry is an emerging technology for direct therapeutic drug monitoring in dried blood spots (DBS). Current DBS methods require manual application of small molecules as internal standards for absolute drug quantification. With industrial standardization in mind, we superseded the manual addition of standard and built a three-layer setup for robust quantification of salicylic acid directly from DBS. We combined a dioctyl sodium sulfosuccinate weave facilitating sample spreading with a cellulose layer for addition of isotope-labeled salicylic acid as internal standard and a filter paper for analysis of the standard-containing sample by DESI-MS. Using this setup, we developed a quantification method for salicylic acid from whole blood with a validated linear curve range from 10 to 2000 mg/L, a relative standard deviation (RSD%) ≤14 %, and determination coefficients of 0.997. The limit of detection (LOD) was 8 mg/L and the lower limit of quantification (LLOQ) was 10 mg/L. Recovery rates in method verification by LC-MS/MS were 97 to 101 % for blinded samples. Most importantly, a study in healthy volunteers after administration of a single dose of Aspirin provides evidence to suggest that the three-layer setup may enable individual pharmacokinetic and endpoint testing following blood collection by finger pricking by patients at home. Taken together, our data suggests that DBS-based quantification of drugs by DESI-MS on pre-manufactured three-layer cartridges may be a promising approach for future near-patient therapeutic drug monitoring.





Similar content being viewed by others
References
Stead AH, Moffat AC (1983) A collection of therapeutic, toxic and fatal blood drug concentrations in man. Hum Toxicol 2(3):437–464
Mullangi R, Sharma K, Srinivas NR (2012) Review of HPLC methods and HPLC methods with mass spectrometric detection for direct determination of aspirin with its metabolite(s) in various biological matrices. Biomed Chromatogr 26(8):906–941. doi:10.1002/bmc.2694
Calabro JJ, Paulus HE (1970) Anti-inflammatory effect of acetylsalicylic acid in rheumatoid arthritis. Clin Orthop Relat Res 71:124–131
Nirogi R, Kandikere V, Mudigonda K, Ajjala D, Suraneni R, Thoddi P (2011) Simultaneous extraction of acetylsalicylic acid and salicylic acid from human plasma and simultaneous estimation by liquid chromatography and atmospheric pressure chemical ionization/tandem mass spectrometry detection. Application to a pharmacokinetic study. Arzneimittelforschung 61(5):301–311. doi:10.1055/s-0031-1296203
Li LP, Feng BS, Yang JW, Chang CL, Bai Y, Liu HW (2013) Applications of ambient mass spectrometry in high-throughput screening. Analyst 138(11):3097–3103. doi:10.1039/c3an00119a
Barfield M, Spooner N, Lad R, Parry S, Fowles S (2008) Application of dried blood spots combined with HPLC-MS/MS for the quantification of acetaminophen in toxicokinetic studies. J Chromatogr B 870(1):32–37. doi:10.1016/j.jchromb.2008.05.025
Spooner N, Lad R, Barfield M (2009) Dried blood spots as a sample collection technique for the determination of pharmacokinetics in clinical studies: considerations for the validation of a quantitative bioanalytical method. Anal Chem 81(4):1557–1563. doi:10.1021/ac8022839
Venter A, Sojka PE, Cooks RG (2006) Droplet dynamics and ionization mechanisms in desorption electrospray ionization mass spectrometry. Anal Chem 78(24):8549–8555. doi:10.1021/ac0615807
Hu Q, Talaty N, Noll RJ, Cooks RG (2006) Desorption electrospray ionization using an Orbitrap mass spectrometer: exact mass measurements on drugs and peptides. Rapid Commun Mass Spectrom 20(22):3403–3408. doi:10.1002/rcm.2757
Friia M, Legros V, Tortajada J, Buchmann W (2012) Desorption electrospray ionization-orbitrap mass spectrometry of synthetic polymers and copolymers. J Mass Spectrom 47(8):1023–1033. doi:10.1002/jms.3057
Wiseman JM, Evans CA, Bowen CL, Kennedy JH (2010) Direct analysis of dried blood spots utilizing desorption electrospray ionization (DESI) mass spectrometry. Analyst 135(4):720. doi:10.1039/b922329k
Liu J, Wang H, Manicke NE, Lin J-M, Cooks RG, Ouyang Z (2010) Development, characterization, and application of paper spray ionization. Anal Chem 82(6):2463–2471. doi:10.1021/ac902854g
Kennedy JH, Wiseman JM (2010) Evaluation and performance of desorption electrospray ionization using a triple quadrupole mass spectrometer for quantitation of pharmaceuticals in plasma. Rapid Commun Mass Spectrom 24(3):309–314. doi:10.1002/rcm.4390
Li W, Tse FL (2010) Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed Chromatogr 24(1):49–65. doi:10.1002/bmc.1367
Parker SP, Khan HI, Cubitt WD (1999) Detection of antibodies to hepatitis C virus in dried blood spot samples from mothers and their offspring in Lahore, Pakistan. J Clin Microbiol 37(6):2061–2063
Wiseman JM, Ifa DR, Zhu Y, Kissinger CB, Manicke NE, Kissinger PT, Cooks RG (2008) Desorption electrospray ionization mass spectrometry: imaging drugs and metabolites in tissues. Proc Natl Acad Sci U S A 105(47):18120–18125. doi:10.1073/pnas.0801066105
Abu-Rabie P, Denniff P, Spooner N, Brynjolffssen J, Galluzzo P, Sanders G (2011) Method of applying internal standard to dried matrix spot samples for use in quantitative bioanalysis. Anal Chem 83(22):8779–8786. doi:10.1021/ac202321q
Liu J, Cooks RG, Ouyang Z (2013) Enabling quantitative analysis in ambient ionization mass spectrometry: internal standard coated capillary samplers. Anal Chem 85(12):5632–5636. doi:10.1021/ac401056q
Przybylski C, Gonnet F, Buchmann W, Daniel R (2012) Critical parameters for the analysis of anionic oligosaccharides by desorption electrospray ionization mass spectrometry. J Mass Spectrom 47(8):1047–1058. doi:10.1002/jms.3052
Green FM, Salter TL, Gilmore IS, Stokes P, O'Connor G (2010) The effect of electrospray solvent composition on desorption electrospray ionisation (DESI) efficiency and spatial resolution. Analyst 135(4):731–737
Katzung BG, Masters SB, Trevor AJ (2009) Basic & clinical pharmacology, 11th edition. McGraw-Hill Medical Publishing Division, New York
Hönes J, Müller P, Surridge, N (2008) The technology behind glucose meters: test strips. Diabetes Technol Ther 10. doi:10.1089/dia.2008.0005
Wagner M, Tonoli D, Varesio E, Hopfgartner G (2014) The use of mass spectrometry to analyze dried blood spots. Mass Spectrom Rev. doi:10.1002/mas.21441
Vuignier K, Guillarme D, Veuthey J-L, Carrupt P-A, Schappler J (2013) High performance affinity chromatography (HPAC) as a high-throughput screening tool in drug discovery to study drug-plasma protein interactions. J Pharm Biomed Anal 74:205–212. doi:10.1016/j.jpba.2012.10.030
de Sain-van der Velden MGM, Diekman EF, Jans JJ, van der Ham M, Prinsen BHCMT, Visser G, Verhoeven-Duif NM (2013) Differences between acylcarnitine profiles in plasma and bloodspots. Mol Genet Metab 110(1–2):116–121. doi:10.1016/j.ymgme.2013.04.008
Tretzel L, Thomas A, Geyer H, Delahaut P, Schanzer W, Thevis M (2015) Determination of Synacthen in dried blood spots for doping control analysis using liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. doi:10.1007/s00216-015-8674-6
Kostic N, Dotsikas Y, Jovic N, Stevanovic G, Malenovic A, Medenica M (2015) Quantitation of pregabalin in dried blood spots and dried plasma spots by validated LC-MS/MS methods. J Pharm Biomed Anal 109:79–84. doi:10.1016/j.jpba.2015.02.023
Meesters RJ, Hooff GP (2013) State-of-the-art dried blood spot analysis: an overview of recent advances and future trends. Bioanalysis 5(17):2187–2208. doi:10.4155/bio.13.175
Stillings M, Havlik I, Chetty M, Clinton C, Schall R, Moodley I, Muir N, Little S (2000) Comparison of the pharmacokinetic profiles of soluble aspirin and solid paracetamol tablets in fed and fasted volunteers. Curr Med Res Opin 16(2):115–124
Thompson JW, Zhang H, Smith P, Hillman S, Moseley MA, Millington DS (2012) Extraction and analysis of carnitine and acylcarnitines by electrospray ionization tandem mass spectrometry directly from dried blood and plasma spots using a novel autosampler. Rapid Commun Mass Spectrom 26(21):2548–2554. doi:10.1002/rcm.6370
Acknowledgments
M.S., K.K., N.F., and V.H. are employees of Roche Diabetes Care GmbH, Mannheim. The company funded the work. The authors thank Herbert Fink for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 731 KB)
Rights and permissions
About this article
Cite this article
Siebenhaar, M., Küllmer, K., de Barros Fernandes, N.M. et al. Personalized monitoring of therapeutic salicylic acid in dried blood spots using a three-layer setup and desorption electrospray ionization mass spectrometry. Anal Bioanal Chem 407, 7229–7238 (2015). https://doi.org/10.1007/s00216-015-8887-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8887-8