Abstract
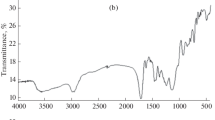
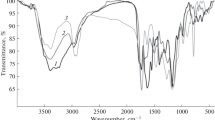
A new restricted access molecularly imprinted polymer coated with bovine serum albumin (RAMIP-BSA) was developed, characterized, and used for direct analysis of chlorpromazine in human plasma samples. The RAMIP-BSA was synthesized using chlorpromazine, methacrylic acid, and ethylene glycol dimethacrylate as template, functional monomer, and cross-linker, respectively. Glycerol dimethacrylate and hydroxy methyl methacrylate were used to promote a hydrophilic surface (high density of hydroxyl groups). Afterward, the polymer was coated with BSA using glutaraldehyde as cross-linker, resulting in a protein chemical shield around it. The material was able to eliminate ca. 99 % of protein when a 44-mg mL−1 BSA aqueous solution was passed through it. The RAMIP-BSA was packed in a column and used for direct analysis of chlorpromazine in human plasma samples in an online column switching high-performance liquid chromatography system. The analytical calibration curve was prepared in a pool of human plasma samples with chlorpromazine concentrations ranging from 30 to 350 μg L−1. The correlation coefficient obtained was 0.995 and the limit of quantification was 30 μg L−1. Intra-day and inter-day precision and accuracy presented variation coefficients and relative errors lower than 15 % and within −15 and 15 %, respectively. The sample throughput was 3 h−1 (sample preparation and chromatographic analysis steps) and the same RAMIP-BSA column was efficiently used for about 90 cycles.






Similar content being viewed by others
References
Dias ACB, Figueiredo EC, Grassi V, Zagatto EAG, Arruda MAZ (2009) Talanta 76:988–996
Figueiredo EC, Sanvido GB, Arruda MAZ, Eberlin MN (2012) Analyst 135:726–730
Magalhães CS, Garcia JS, Lopes AS, Figueiredo EC, Arruda MAZ (2007) In: Arruda MAZ (ed) Trends in sample preparation. Nova Science, New York
Figueiredo EC, de Oliveira DM, de Siqueira MEPB, Arruda MAZ (2009) Anal Chim Acta 635:102–107
Vieira AC, Zampieri RA, de Siqueira MEPB, Martins I, Figueiredo EC (2012) Analyst 137:2462–2469
Vitor RV, Martins MCG, Figueiredo ECF, Martins I (2011) Anal Bioanal Chem 400:2109–2117
Figueiredo EC, Sparrapan R, Sanvido GB, Santos MG, Arruda MAZ, Eberlin MN (2011) Analyst 136:3753–3757
Franqui LS, Vieira AC, Maia PP, Figueiredo EC (2012) Quim Nova 35:1577–1581
Golsefidi MA, Es'haghi Z, Sarafraz-Yazdi A (2012) J Chromatogr A 1229:24–29
Feng JJ, Sun M, Li JB, Liu X, Jiang SX (2012) J Chromatogr A 1227:54–59
Lee TP, Saad B, Khayoon WS, Salleh B (2012) Talanta 88:129–135
Wang S, Wei J, Hao TT, Guo ZT (2012) J Electroanal Chem 664:146–151
Hu YL, Li JW, Hu YF, Li GK (2010) Talanta 82:464–470
Prieto A, Vallejo A, Zuloaga O, Paschke A, Sellergen B, Schillinger E, Schrader S, Moder M (2011) Anal Chim Acta 703:41–51
Prieto A, Schrader S, Bauer C, Moder M (2011) Anal Chim Acta 685:146–152
Zhang ZM, Tan W, Hu YL, Li GK, Zan S (2012) Analyst 137:968–977
Souverain S, Rudaz S, Veuthey J (2004) J Chromatogr B 801:141–156
Haginaka J, Sanbe H (2000) Anal Chem 72:5206–5210
Haginaka J, Takehira H, Hosaya K, Tanaka N (1999) J Chromatogr A 849:331–339
Sanbe H, Haginaka J (2003) Analyst 128:593–597
Puoci F, Iemma F, Cirillo G, Curcio M, Oarisi OI, Spizzirri UG, Picci N (2009) Eur Polym J 45:1634–1640
Parisi OI, Cirillo G, Curcio M, Puoci F, Iemma F, Spizzirri UG, Picci N (2010) J Polym Res 17:355–362
Hua K, Zhang L, Zhang Z, Guo Y, Guo T (2011) Acta Biomater 7:3086–3096
dos Santos-Neto AJ, Fernandes C, Rodrigues JC, Alves C, Lanças FM (2008) J Sep Sci 31:78–85
Barreiro JC, Vanzolini KL, Cass QB (2011) J Chromatogr A 1218:2865–2870
Santos Neto AJ, Rodrigues JC, Fernandes C, Titato GM, Alves C, Lanças FM (2006) J Chromatogr A 1105:71–76
Cassiano NM, Lima VV, Oliveira RV, Pietro AC, Cass QB (2006) Anal Bioanal Chem 384:1462–1469
Gomes RF, Cassiano NM, Pedrazzoli J, Cass QB (2010) Chirality 22:35–41
Menezes ML, Felix G (1998) J Liq Chromatogr Relat Technol 21:2863–2871
Love JN, Smith JA, Simmons R (2006) J Emerg Med 31:53–59
Radhika M, Palanivelu K (2006) J Hazard Mater 138:116–124
Gregg SJ, Sing KSW (1982) Adsorption, surface area and porosity. Academic, London
Sobhi HR, Yamini Y, Abadi RHHB (2007) J Pharm Biomed Anal 45:769–774
Shabir GA (2003) J Chromatogr A 987:57–66
Kirchherr H, Kühn-Velten WN (2006) J Chromatogr B 843:100–113
United States Food and Drug Administration (US FDA) (2001) Guidance for industry. Bioanalytical method validation. FDA Center for Drug Evaluation and Research, Rockville
Hoshina K, Horiyama S, Matsunaga H, Haginaka J (2011) J Pharm Biomed Anal 55:916–922
Xu W, Su S, Jiang P, Wang H, Dong X, Zhang M (2010) J Chromatogr A 1217:7198–7207
Acknowledgments
We thank the “Fundação de Amparo à Pesquisa do Estado de Minas Gerais” (Belo Horizonte, Brazil; projects CDS-APQ-01612-10 and CDS-APQ-01323-09), “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (Brasília, Brazil), and “Fundação de Amparo à Pesquisa do Estado de São Paulo” (São Paulo, Brazil; process 2007/50970-5).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 159 kb)
Rights and permissions
About this article
Cite this article
de Oliveira Isac Moraes, G., da Silva, L.M.R., dos Santos-Neto, Á.J. et al. A new restricted access molecularly imprinted polymer capped with albumin for direct extraction of drugs from biological matrices: the case of chlorpromazine in human plasma. Anal Bioanal Chem 405, 7687–7696 (2013). https://doi.org/10.1007/s00216-013-7275-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7275-5