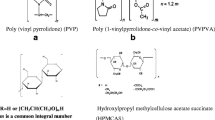
Abstract
In this work, Taylor dispersion analysis and capillary electrophoresis were used to characterize the size and charge of polymeric drug delivery nanogels based on polyglutamate chains grafted with hydrophobic groups of vitamin E. The hydrophobic vitamin E groups self-associate in water to form small hydrophobic nanodomains that can incorporate small drugs or therapeutic proteins. Taylor dispersion analysis is well suited to determine the weight average hydrodynamic radius of nanomaterials and to get information on the size polydispersity of polymeric samples. The effective charge was determined either from electrophoretic mobility and hydrodynamic radius using electrophoretic modeling (three different approaches were compared), or by indirect UV detection in capillary electrophoresis. The influence of vitamin E hydrophobicity on the polymer effective charge has been studied. The presence of vitamin E leads to a drastic decrease in polymer effective charge in comparison to non-modified polyglutamate. Finally, the electrophoretic behavior of polyglutamate backbone grafted with hydrophobic vitamin E (pGVE) nanogels according to the ionic strength was investigated using the recently proposed slope plot approach. It was deduced that the pGVE nanogels behave electrophoretically as polyelectrolytes which is in good agreement with the high water content of the nanogels.

Size and charge characterization of polyglutamate-based drug delivery systems by Taylor dispersion analysis, indirect UV detection and the 'Slope-plot' approach





Similar content being viewed by others
References
Duncan R (2003) The dawning era of polymer therapeutics. Nat Rev Drug Discov 2(5):347–360
Duncan R, Ringsdorf H, Satchi-Fainaro R (2006) Polymer therapeutics: polymers as drugs, drug and protein conjugates and gene delivery systems: past, present and future opportunities. Adv Polym Sci 192:1–8. doi:10.1007/12_037
Jeong B, Bae YH, Kim SW (2000) Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J Controlled Release 63:155–163
Aurand ER, Lampe KJ, Bjugstad KB (2012) Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci Res 72(3):199–213
Liechty WB, Kryscio DR, Slaughter BV, Peppas NA (2010) Polymers for drug delivery systems. Annu Rev Chem Biomol Eng 1:149–173
Allen TM, Cullis PR (2004) Drug delivery systems: entering the mainstream. Science 303(5665):1818–1822
Liechty WB, Peppas NA (2012) Expert opinion: responsive polymer nanoparticles in cancer therapy. Eur J Pharm Biopharm 80(2):241–246
Couvreur P, Dubernet C, Puisieux F (1995) controlled drug-delivery with nanoparticles—current possibilities and future trends. Eur J Pharm Biopharm 41(1):2–13
Brannon-Peppas L, Blanchette JO (2004) Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev 56(11):1649–1659
Jewell CM, Zhang J, Fredin NJ, Lynn DM (2005) Multilayered polyelectrolyte films promote the direct and localized delivery of DNA to cells. J Controlled Release 106:214–223
Raemdonck K, Demeester J, De Smedt S (2009) Advanced nanogel engineering for drug delivery. Soft Matter 5(4):707–715
Peppas NA, Bures P, Leobandung W, Ichikawa H (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 50(1):27–46
Lin C-C, Anseth K (2009) PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res 26(3):631–643
Fisher O, Kim T, Dietz S, Peppas N (2009) Enhanced core hydrophobicity, functionalization and cell penetration of polybasic nanomatrices. Pharm Res 26(1):51–60
Kafka AP, Kleffmann T, Rades T, McDowell A (2009) Histidine residues in the peptide D-Lys(6)-GnRH: potential for copolymerization in polymeric nanoparticles. Mol Pharm 6(5):1483–1491
Sahiner N, Ilgin P (2010) Synthesis and characterization of soft polymeric nanoparticles and composites with tunable properties. J Polym Sci Pol Chem 48(22):5239–5246
Chan YP, Meyrueix R, Kravtzoff R, Nicolas F, Lundstrom K (2007) Review on Medusa (R): a polymer-based sustained release technology for protein and peptide drugs. Expert Opin Drug Deliv 4(4):441–451
Sharma U, Gleason NJ, Carbeck JD (2005) Diffusivity of solutes measured in glass capillaries using Taylor’s analysis of dispersion and a commercial CE instrument. Anal Chem 77(3):806–813
Ye F, Jensen H, Larsen SW, Yaghmur A, Larsen C, Ostergaard J (2012) Measurement of drug diffusivities in pharmaceutical solvents using Taylor dispersion analysis. J Pharm Biomed Anal 61:176–183
Cottet H, Biron JP, Cipelletti L, Matmour R, Martin M (2010) Determination of individual diffusion coefficients in evolving binary mixtures by Taylor dispersion analysis: application to the monitoring of polymer reaction. Anal Chem 82(5):1793–1802
Le Saux T, Cottet H (2008) Size-based characterization by the coupling of capillary electrophoresis to Taylor dispersion analysis. Anal Chem 80(5):1829–1832
Leclercq L, Cottet H (2012) Fast characterization of polyelectrolyte complexes by inline coupling of capillary electrophoresis to Taylor dispersion analysis. Anal Chem 84(3):1740–1743
d’Orlye F, Varenne A, Gareil P (2008) Determination of nanoparticle diffusion coefficients by Taylor dispersion analysis using a capillary electrophoresis instrument. J Chromatogr A 1204(2):226–232
Cottet H, Martin M, Papillaud A, Souaid E, Collet H, Commeyras A (2007) Determination of dendrigraft poly-L-lysine diffusion coefficients by Taylor dispersion analysis. Biomacromolecules 8(10):3235–3243
Hawe A, Hulse WL, Jiskoot W, Forbes RT (2011) Taylor dispersion analysis compared to dynamic light scattering for the size analysis of therapeutic peptides and proteins and their aggregates. Pharm Res 28(9):2302–2310
Ostergaard J, Jensen H (2009) Simultaneous evaluation of ligand binding properties and protein size by electrophoresis and Taylor dispersion in capillaries. Anal Chem 81(20):8644–8648
Hulse WL, Forbes RT (2011) A nanolitre method to determine the hydrodynamic radius of proteins and small molecules by Taylor dispersion analysis. Int J Pharm 411(1–2):64–68
Hulse W, Forbes R (2011) A Taylor dispersion analysis method for the sizing of therapeutic proteins and their aggregates using nanolitre sample quantities. Int J Pharm 416(1):394–397
Franzen U, Vermehren C, Jensen H, Ostergaard J (2011) Physicochemical characterization of a PEGylated liposomal drug formulation using capillary electrophoresis. Electrophoresis 32(6–7):738–748
Bello MS, Rezzonico R, Righetti PG (1994) Use of taylor-aris dispersion for measurement of a solute diffusion-coefficient in thin capillaries. Science 266(5186):773–776
Cottet H, Biron JP, Martin M (2007) Taylor dispersion analysis of mixtures. Anal Chem 79(23):9066–9073
Gitlin I, Carbeck JD, Whitesides GM (2006) Why are proteins charged? Networks of charge-charge interactions in proteins measured by charge ladders and capillary electrophoresis. Angew Chem Int Ed 45(19):3022–3060
Seyrek E, Dubin PL, Henriksen J (2007) Nonspecific electrostatic binding characteristics of the heparin-antithrombin interaction. Biopolymers 86(3):249–259
Manning GS (1969) Limiting laws and counterion condensation in polyelectrolyte solutions. I. Colligative properties. J Chem Phys 51(3):924–933
Chepelianskii A, Mohammad-Rafiee F, Trizac E, Raphael E (2009) On the effective charge of hydrophobic polyelectrolytes. J Phys Chem B 113(12):3743–3749
Essafi W, Lafuma F, Baigl D, Williams CE (2005) Anomalous counterion condensation in salt-free hydrophobic polyelectrolyte solutions: osmotic pressure measurements. Europhys Lett 71(6):938–944
Allison SA, Perrin C, Cottet H (2011) Modeling the electrophoresis of oligolysines. Electrophoresis 32(20):2788–2796
Vuletic T, Babic SD, Grgicin D, Aumiler D, Radler J, Livolant F, Tomic S (2011) Manning free counterion fraction for a rodlike polyion: aqueous solutions of short DNA fragments in presence of very low added salt. Physical Review E 83(4):8–16
Boisvert JP, Malgat A, Pochard I, Daneault C (2002) Influence of the counter-ion on the effective charge of polyacrylic acid in dilute condition. Polymer 43(1):141–148
Combet J, Rawiso M, Rochas C, Hoffmann S, Boue F (2011) Structure of polyelectrolytes with mixed monovalent and divalent counterions: SAXS measurements and Poisson–Boltzmann analysis. Macromolecules 44(8):3039–3052
Bohme U, Scheler U (2003) Effective charge of poly(styrenesulfonate) and ionic strength—an electrophoresis NMR investigation. Colloids Surf A 222(1–3):35–40
Anik N, Airiau M, Labeau MP, Vuong CT, Reboul J, Lacroix-Desmazes P, Gerardin C, Cottet H (2009) Determination of polymer effective charge by indirect UV detection in capillary electrophoresis: toward the characterization of macromolecular architectures. Macromolecules 42(7):2767–2774
Pyell U, Bucking W, Huhn C, Herrmann B, Merkoulov A, Mannhardt J, Jungclas H, Nann T (2009) Calibration-free concentration determination of charged colloidal nanoparticles and determination of effective charges by capillary isotachophoresis. Anal Bioanal Chem 395(6):1681–1691
Agnihotri SM, Ohshima H, Terada H, Tomoda K, Makino K (2009) Electrophoretic mobility of colloidal gold particles in electrolyte solutions. Langmuir 25(8):4804–4807
Oukacine F, Morel A, Cottet H (2011) Characterization of carboxylated nanolatexes by capillary electrophoresis. Langmuir 27(7):4040–4047
Xin Y, Mitchell H, Cameron H, Allison SA (2006) Modeling the electrophoretic mobility and diffusion of weakly charged peptides. J Phys Chem B 110(2):1038–1045
Li SK, Liddell MR, Wen H (2011) Effective electrophoretic mobilities and charges of anti-VEGF proteins determined by capillary zone electrophoresis. J Pharm Biomed Anal 55(3):603–607
Ibrahim A, Ohshima H, Allison SA, Cottet H (2012) Determination of effective charge of small ions, polyelectrolytes and nanoparticles by capillary electrophoresis. J Chromatogr A 1247:154–164
Taylor G (1953) Dispersion of soluble matter in solvent flowing slowly through a tube. Proc R Soc London, Ser A 219(1137):186–203
Aris R (1956) On the dispersion of a solute in a fluid flowing through a tube. Proc R Soc London, Ser A 235(1200):67–77
Chamieh J, Oukacine F, Cottet H (2012) Taylor dispersion analysis with two detection points on a commercial capillary electrophoresis apparatus. J Chromatogr A 1235:174–177
Taylor G (1954) Conditions under which dispersion of a solute in a stream of solvent can be used to measure molecular diffusion. Proc R Soc London, Ser A 225(1163):473–477
Chamieh J, Cottet H (2012) Comparison of single and double detection points Taylor dispersion analysis for monodisperse and polydisperse samples. J Chromatogr A 1241:123–127
Einstein A (1906) A new determination of the molecular dimensions. Ann Phys 19(2):289–306
Stokes G (1966) Vol Cambridge Philos Trans 1851;9:8. Reprinted in: Mathematical and Physical Papers, 2nd edition. Johnson Reprint Corp., New York
Pitts E (1953) An extension of the theory of the conductivity and viscosity of electrolyte solutions. Proc R Soc London, Ser A 217(1128):43–70
Plasson R, Cottet H (2005) Determination of homopolypeptide conformational changes by the modeling of electrophoretic mobilities. Anal Chem 77(18):6047–6054
Friedl W, Reijenga JC, Kenndler E (1995) Ionic-strength and charge number correction for mobilities of multivalent organic-anions in capillary electrophoresis. J Chromatogr A 709(1):163–170
Long D et al (1996) A Zimm model for polyelectrolytes in an electric field. J Phys Condens Matter 8(47):9471–9475
Ohshima H (2001) Approximate analytic expression for the electrophoretic mobility of a spherical colloidal particle. J Colloid Interface Sci 239(2):587–590
Makino K, Ohshima H (2010) Electrophoretic mobility of a colloidal particle with constant surface charge density. Langmuir 26(23):18016–18019
Yoon BJ, Kim S (1989) Electrophoresis of spheroidal particles. J Colloid Interface Sci 128(1):275–288
Allison SA, Pei HX, Baek S, Brown J, Lee MY, Nguyen V, Twahir UT, Wu HF (2010) The dependence of the electrophoretic mobility of small organic ions on ionic strength and complex formation. Electrophoresis 31(5):920–932
Ohshima H (1994) A simple expression for henrys function for the retardation effect in electrophoresis of spherical colloidal particles. J Colloid Interface Sci 168(1):269–271
O’Brien RW, White LR (1978) Electrophoretic mobility of a spherical colloidal particle. J Chem Soc Faraday Trans 2(74):1607–1626
Doble P, Andersson P, Haddad PR (1997) Determination and prediction of transfer ratios for anions in capillary zone electrophoresis with indirect UV detection. J Chromatogr A 770(1–2):291–300
Hjerten S, Elenbring K, Kilar F, Liao JL, Chen AJC, Siebert CJ, Zhu MD (1987) Carrier-free zone electrophoresis, displacement electrophoresis and isoelectric-focusing in a high-performance electrophoresis apparatus. J Chromatogr 403:47–61
Johns C, Macka M, Haddad PR (2003) Enhancement of detection sensitivity for indirect photometric detection of anions and cations in capillary electrophoresis. Electrophoresis 24(12–13):2150–2167
Kohlrausch F (1897) Ueber Concentrations-Verschiebungen durch Electrolyse im Inneren von Lösungen und Lösungsgemischen. Ann Phys 298(10):209–239
Nishio T (1998) Monte Carlo studies on potentiometric titration of poly(glutamic acid). Biophys Chem 71(2–3):173–184
Holm C, Joanny JF, Kremer K, Netz RR, Reineker P, Seidel C, Vilgis TA, Winkler RG (2004) Polyelectrolyte theory. In: Schmidt M (ed) Polyelectrolytes with defined molecular architecture. Springer, Berlin, pp 67–111
Ibrahim A, Allison SA, Cottet H (2012) Extracting information from the ionic strength dependence of electrophoretic mobility by use of the slope plot. Anal Chem 84:9422–9430
Acknowledgments
H.C. gratefully acknowledges the support from the Région Languedoc-Roussillon for the fellowship “Chercheurs d’Avenir” and from the Institut Universitaire de France.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 212 kb)
Rights and permissions
About this article
Cite this article
Ibrahim, A., Meyrueix, R., Pouliquen, G. et al. Size and charge characterization of polymeric drug delivery systems by Taylor dispersion analysis and capillary electrophoresis. Anal Bioanal Chem 405, 5369–5379 (2013). https://doi.org/10.1007/s00216-013-6972-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6972-4