Abstract
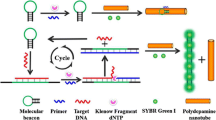
Because small molecules can be beneficial or toxic in biology and the environment, specific and sensitive detection of small molecules is one of the most important objectives of the scientific community. In this study, new signal amplification assays for detection of small molecules based on Mg2+-dependent DNAzyme were developed. A cleavable DNA substrate containing a ribonucleotide, the ends of which were labeled with black hole quencher (BHQ) and 6-carboxyfluorescein (FAM), was used for fluorescence detection. When the small molecule of interest is added to the assay solution, the Mg2+-dependent DNAzyme is activated, facilitating hybridization between the Mg2+-dependent DNAzyme and the DNA substrate. Binding of the substrate to the DNAzyme structure results in hydrolytic cleavage of the substrate in the presence of Mg2+ ions. The fluorescence signal was amplified by continuous cleavage of the enzyme substrate. Ochratoxin A (OTA) and adenosine triphosphate (ATP) were used as model analytes in these experiments. This method can detect OTA specifically with a detection limit as low as 140 pmol L−1 and detect ATP specifically with a detection limit as low as 13 nmol L−1. Moreover, this method is potentially extendable to detection of other small molecules which are able to dissociate the aptamer from the DNAzyme, leading to activation of the DNAzyme.







Similar content being viewed by others
References
Song P, Xiang Y, Xing H, Zhou Z, Tong A, Lu Y (2012) Label-free catalytic and molecular beacon containing an abasic site for sensitive fluorescent detection of small inorganic and organic molecules. Anal Chem 84(6):2916–2922. doi:10.1021/ac203488p
Ho D, Dose C, Albrecht CH, Severin P, Falter K, Dervan PB, Gaub HE (2009) Quantitative detection of small molecule/DNA complexes employing a force-based and label-free DNA-microarray. Biophys J 96(11):4661–4671. doi:10.1016/j.bpj.2009.02.059
Matsui J, Akamatsu K, Hara N, Miyoshi D, Nawafune H, Tamaki K, Sugimoto N (2005) SPR sensor chip for detection of small molecules using molecularly imprinted polymer with embedded gold nanoparticles. Anal Chem 77(13):4282–4285. doi:10.1021/ac050227i
Xia F, Zuo X, Yang R, Xiao Y, Kang D, Vallee-Belisle A, Gong X, Yuen JD, Hsu BBY, Heeger AJ, Plaxco KW (2010) Colorimetric detection of DNA, small molecules, proteins, and ions using unmodified gold nanoparticles and conjugated polyelectrolytes. Nat Acad Sci USA 107(24):10837–10841. doi:10.1073/pnas.1005632107
Zheng D, Zou R, Lou X (2012) Label-free fluorescent detection of ions, proteins, and small molecules using structure-switching aptamers, SYBR gold, and exonuclease I. Anal Chem 84(8):3554–3560. doi:10.1021/ac300690r
Gianneschi NC, Nguyen ST, Mirkin CA (2005) Signal amplification and detection via a supramolecular allosteric catalyst. J Am Chem Soc 127(6):1644–1645. doi:10.1021/ja0437306
Masar MS III, Gianneschi NC, Oliveri CG, Stern CL, Nguyen ST, Mirkin CA (2007) Allosterically regulated supramolecular catalysis of acyl transfer reactions for signal amplification and detection of small molecules. J Am Chem Soc 129(33):10149–10158. doi:10.1021/ja0711516
Baker MS, Phillips ST (2011) A two-component small molecule system for activity-based detection and signal amplification: application to the visual detection of threshold levels of Pd(II). J Am Chem Soc 133(14):5170–5173. doi:10.1021/ja108347d
Tang J, Tang D, Zhou J, Yang H, Chen G (2012) Nuclease cleavage-assisted target recycling for signal amplification of free-label impedimetric aptasensors. Chem Comm 48(20):2627–2629. doi:10.1039/c2cc17536c
Patolsky F, Lichtenstein A, Willner I (2001) Detection of single-base DNA mutations by enzyme-amplified electronic transduction. Nature Biotechnol 19(3):253–257. doi:10.1038/85704
Polsky R, Gill R, Kaganovsky L, Willner I (2006) Nucleic acid-functionalized Pt nanoparticles: catalytic labels for the amplified electrochemical detection of biomolecules. Anal Chem 78(7):2268–2271. doi:10.1021/ac0519864
Zhou LP, Yang J, Estavillo C, Stuart JD, Schenkman JB, Rusling JF (2003) Toxicity screening by electrochemical detection of DNA damage by metabolites generated in situ in ultrathin DNA-enzyme films. J Am Chem Soc 125(5):1431–1436. doi:10.1021/ja0290274
Shimoda J, Maruyama T, Kitaoka M, Kamiya N, Goto M (2011) DNA-enzyme conjugate with a weak inhibitor that can specifically detect thrombin in a homogeneous medium. Anal Biochem 414(1):103–108. doi:10.1016/j.ab.2011.02.035
Lu L-M, Zhang X-B, Kong R-M, Yang B, Tan W (2011) A ligation-triggered DNAzyme cascade for amplified fluorescence detection of biological small molecules with zero-background signal. J Am Chem Soc 133(30):11686–11691. doi:10.1021/ja203693b
Zaborowska Z, Schubert S, Kurreck J, Erdmann VA (2005) Deletion analysis in the catalytic region of the 10-23 DNA enzyme. FEBS Lett 579(2):554–558. doi:10.1016/j.febslet.2004.12.008
Elbaz J, Moshe M, Shlyahovsky B, Willner I (2009) Cooperative multicomponent self-assembly of nucleic acid structures for the activation of dnazyme cascades: a paradigm for DNA sensors and aptasensors. Chem Eur J 15(14):3411–3418. doi:10.1002/chem.200802004
Shimron S, Wang F, Orbach R, Willner I (2012) Amplified detection of DNA through the enzyme-free autonomous assembly of hemin/G-quadruplex DNAzyme nanowires. Anal Chem 84(2):1042–1048. doi:10.1021/ac202643y
Osborne SE, Ellington AD (1997) Nucleic acid selection and the challenge of combinatorial chemistry. Chem Rev 97(2):349–370. doi:10.1021/cr960009c
Shimron S, Elbaz J, Henning A, Willner I (2010) Ion-induced DNAzyme switches. Chem Comm 46(19):3250–3252. doi:10.1039/b926003j
Wang F, Elbaz J, Teller C, Willner I (2011) Amplified detection of DNA through an autocatalytic and catabolic DNAzyme-mediated process. Angew Chem Int Edit 50(1):295–299. doi:10.1002/anie.201005246
Xiao Y, Pavlov V, Niazov T, Dishon A, Kotler M, Willner I (2004) Catalytic beacons for the detection of DNA and telomerase activity. J Am Chem Soc 126(24):7430–7431. doi:10.1021/ja031875r
Teller C, Shimron S, Willner I (2009) Aptamer-DNAzyme hairpins for amplified biosensing. Anal Chem 81(21):9114–9119. doi:10.1021/ac901773b
Guo Z, Ren J, Wang J, Wang E (2011) Single-walled carbon nanotubes based quenching of free FAM-aptamer for selective determination of ochratoxin A. Talanta 85(5):2517–2521. doi:10.1016/j.talanta.2011.08.015
Pfohl-Leszkowicz A, Manderville RA (2007) Ochratoxin A: an overview on toxicity and carcinogenicity in animals and humans (vol 51, pg 61, 2007). Mol Nutr Food Res 51:1192. doi:10.1002/mnfr.200600137
Dall’Asta C, Galaverna G, Bertuzzi T, Moseriti A, Pietri A, Dossena A, Marchelli R (2011) Occurrence of ochratoxin A in raw ham muscle, salami and dry-cured ham from pigs fed with contaminated diet (vol 120, pg 978, 2010). Food Chem 126(1):301. doi:10.1016/j.foodchem.2010.08.061
Assaf H, Azouri H, Pallardy M (2004) Ochratoxin A induces apoptosis in human lymphocytes through down regulation of Bcl-x(L). Toxicol Sci 79(2):335–344. doi:10.1093/toxsci/kfh123
Juan C, Molto JC, Lino CM, Manes J (2008) Determination of ochratoxin A in organic and non-organic cereals and cereal products from Spain and Portugal. Food Chem 107(1):525–530. doi:10.1016/j.foodchem.2007.08.019
Sheng LF, Ren JT, Miao YQ, Wang JH, Wang EK (2011) PVP-coated graphene oxide for selective determination of ochratoxin A via quenching fluorescence of free aptamer. Biosens Bioelectron 26(8):3494–3499. doi:10.1016/j.bios.2011.01.032
Asuncion Alonso-Lomillo M, Dominguez-Renedo O, del Torno-de Roman L, Julia Arcos-Martinez M (2011) Horseradish peroxidase-screen printed biosensors for determination of Ochratoxin A. Anal Chim Acta 688(1):49–53. doi:10.1016/j.aca.2011.01.003
Belakova S, Benesova K, Mikulikova R, Svoboda Z (2011) Determination of ochratoxin A in brewing materials and beer by ultra performance liquid chromatography with fluorescence detection. Food Chem 126(1):321–325. doi:10.1016/j.foodchem.2010.10.062
Chen J, Fang Z, Liu J, Zeng L (2012) A simple and rapid biosensor for ochratoxin A based on a structure-switching signaling aptamer. Food Control 25(2):555–560. doi:10.1016/j.foodcont.2011.11.039
Liang A, Ouyang H, Jiang Z (2011) Resonance scattering spectral detection of trace ATP based on label-free aptamer reaction and nanogold catalysis. Analyst 136(21):4514–4519. doi:10.1039/c1an15542c
Zeng X, Zhang X, Yang W, Jia H, Li Y (2012) Fluorescence detection of adenosine triphosphate through an aptamer-molecular beacon multiple probe. Anal Biochem 424(1):8–11. doi:10.1016/j.ab.2012.01.021
Cai L, Chen Z-Z, Dong X-M, Tang H-W, Pang D-W (2011) Silica nanoparticles based label-free aptamer hybridization for ATP detection using hoechst33258 as the signal reporter. Biosens Bioelectron 29(1):46–52. doi:10.1016/j.bios.2011.07.064
He H-Z, Ma VP-Y, Leung K-H, Chan DS-H, Yang H, Cheng Z, Leung C-H, Ma D-L (2012) A label-free G-quadruplex-based switch-on fluorescence assay for the selective detection of ATP. Analyst 137(7):1538–1540. doi:10.1039/c2an15999f
Yang C, Lates V, Prieto-Simon B, Marty J-L, Yang X (2012) Aptamer-DNAzyme hairpins for biosensing of Ochratoxin A. Biosens Bioelectron 32(1):208–212. doi:10.1016/j.bios.2011.12.011
He H-Z, Ma VP-Y, Leung K-H, Chan DS-H, Yang H, Cheng Z, Leung C-H, Ma D-L (2012) A label-free G-quadruplex-based switch-on fluorescence assay for the selective detection of ATP. Analyst 137(7):1538–1540. doi:10.1039/c2an15999f
Li F, Du Z, Yang L, Tang B (2013) Selective and sensitive turn-on detection of adenosine triphosphate and thrombin based on bifunctional fluorescent oligonucleotide probe. Biosens Bioelectron 41:907–910. doi:10.1016/j.bios.2012.10.007
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos 21190040 and 21275137), Start Funding from Changchun Institute of Applied Chemistry (CAS), and Supporting Funding for Creative Young Scientists (CAS).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 24 kb)
Rights and permissions
About this article
Cite this article
Guo, Z., Wang, J. & Wang, E. Signal-amplification detection of small molecules by use of Mg2+- dependent DNAzyme. Anal Bioanal Chem 405, 4051–4057 (2013). https://doi.org/10.1007/s00216-013-6788-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6788-2