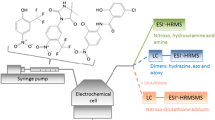
Abstract
Diclofenac is a frequently prescribed drug for rheumatic diseases and muscle pain. In rare cases, it may be associated with a severe hepatotoxicity. In literature, it is discussed whether this toxicity is related to the oxidative phase I metabolism, resulting in electrophilic quinone imines, which can subsequently react with nucleophiles present in the liver in form of glutathione or proteins. In this work, electrochemistry coupled to mass spectrometry is used as a tool for the simulation of the oxidative pathway of diclofenac. Using this purely instrumental approach, diclofenac was oxidized in a thin layer cell equipped with a boron doped diamond working electrode. Sum formulae of generated oxidation products were calculated based on accurate mass measurements with deviations below 2 ppm. Quinone imines from diclofenac were detected using this approach. It could be shown for the first time that these quinone imines do not react with glutathione exclusively but also with larger molecules such as the model protein β-lactoglobulin A. A tryptic digest of the generated drug–protein adduct confirms that the protein is modified at the only free thiol-containing peptide. This simple and purely instrumental set-up offers the possibility of generating reactive metabolites of diclofenac and to assess their reactivity rapidly and easily.








Similar content being viewed by others
References
Helfgott SM, Sandberg-Cook J, Zakim D, Nestler J (1990) JAMA 264:2660–2662
Stierlin J, Faigle W (1979) Xenobiotica 9:611–621
Tang W (2003) Curr Drug Metab 4:319–329
Pumford NR, Myers TG, Davila JC, Highet RJ, Pohl LR (1993) Chem Res Toxicol 6:147–150
Hargus SJ, Amouzedeh HR, Pumford NR, Myers TG, McCoy SC, Pohl LR (1994) Chem Res Toxicol 7:575–582
Shen S, Hargus SJ, Martin BM, Pohl LR (1997) Chem Res Toxicol 10:420–423
Shen S, Marchick MR, Davis MR, Doss GA, Pohl LR (1999) Chem Res Toxicol 12:214–222
Bort R, Macé K, Boobis A, Gómez-Lechón MJ, Pfeifer A, Castell J (1999) Biochem Pharmacol 58:787–796
Jurima-Romet M, Crawford K, Huang HS (1994) Toxicol In Vitro 8:55–66
Kretz-Rommel A, Boelsterli UA (1993) Toxicol Appl Pharmacol 120:155–161
Zhou S (2003) J Chromatogr B Analyt Technol Biomed Life Sci 797:63–90
Jurva U, Wikstrom HV, Weidolf L, Bruins AP (2003) Rapid Commun Mass Spectrom 17:800–810
Lohmann W, Baumann A, Karst U (2010) LC GC Europe 23:1–7
Nouri-Nigjeh E, Bischoff R, Bruins AP, Permentier HP (2011) Curr Drug Metab 12:359–371
Baumann A, Karst U (2010) Expert Opin Drug Metab Toxicol 6:715–731
Permentier HP, Bruins AP, Bischoff R (2008) Mini Rev Med Chem 8:46–56
Zettersten C, Lomoth R, Hammarström L, Sjöberg PJR, Nyholm L (2006) J Electroanal Chem 590:90–99
Lohmann W, Hayen H, Karst U (2008) Anal Chem 80:9714–9719
Madsen KG, Skonberg C, Jurva U, Cornett C, Hansen SH, Johansen TN, Olsen J (2008) Chem Res Toxicol 21:1107–1119
Torii S, Tanaka H (2001) In: Hammerich O, Lund H (eds) Organic electrochemistry, 4th edn. Marcel Dekker, New York
Madsen KG, Olsen J, Skonberg C, Hansen SH, Jurva U (2007) Chem Res Toxicol 20:821–831
Baumann A, Lohmann W, Schubert B, Oberacher H, Karst U (2009) J Chromatogr A 1216:3192–3198
Tang W, Stearns RA, Wang RW, Chiu SH, Baillie TA (1999) Chem Res Toxicol 12:192–199
Dieckhaus CM, Fernandez-Metzler CL, King R, Krolikowski PH, Baillie TA (2005) Chem Res Toxicol 18:630–638
Acknowledgement
The German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt, DBU, Osnabrück, Germany) is gratefully acknowledged for financial support in form of a Ph.D. scholarship for Helene Faber.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special paper collection on Electrochemistry–Mass Spectrometry with guest editors Uwe Karst and Martin Vogel.
Rights and permissions
About this article
Cite this article
Faber, H., Melles, D., Brauckmann, C. et al. Simulation of the oxidative metabolism of diclofenac by electrochemistry/(liquid chromatography/)mass spectrometry. Anal Bioanal Chem 403, 345–354 (2012). https://doi.org/10.1007/s00216-011-5665-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5665-0