Abstract
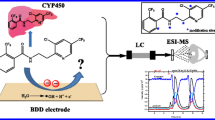
Small-molecule metabolism has been extensively studied in the past decades, notably driven by the development of new pharmaceutical ingredients. The understanding of metabolism is critical to the anticipation of reactive metabolite formation in vivo that is often associated with toxicity. Electrochemistry has been proposed to simulate the oxidoreductive metabolism reaction catalyzed by cytochrome P450, a family of microsomal enzymes strongly involved in xenobiotic metabolism. The implementation of an electrochemical cell online with mass spectrometry allows for the fast formation and identification of the reaction end products. This study discusses the ability of the synthetic electrochemical approach to simulate a complex lactamization reaction that involves the formation of reactive metabolites. Aristolochic acid I was used as a model molecule to evaluate the ability of electrochemical simulation to generate nitroso, hydroxylamine, N-hydroxylactam, lactam, and nitrenium ion metabolites.






Similar content being viewed by others
References
Guengerich FP. Cytochrome p450 and chemical toxicology. Chem Res Toxicol. 2008;21(1):70–83. https://doi.org/10.1021/tx700079z.
Guengerich FP, Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chem Res Toxicol. 1991;4(4):391–407. https://doi.org/10.1021/tx00022a001.
Yamazaki H, Shibata A, Suzuki M, Nakajima M, Shimada N, Guengerich FP, Yokoi T. Oxidation of troglitazone to a quinone-type metabolite catalyzed by cytochrome P-450 2C8 and P-450 3A4 in human liver microsomes. Drug Metab Dispos. 1999;27(11):1260.
Patten CJ, Thomas PE, Guy RL, Lee M, Gonzalez FJ, Guengerich FP, Yang CS. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol. 1993;6(4):511–8. https://doi.org/10.1021/tx00034a019.
Park BK, Boobis A, Clarke S, Goldring CE, Jones D, Kenna JG, Lambert C, Laverty HG, Naisbitt DJ, Nelson S, Nicoll-Griffith DA, Obach RS, Routledge P, Smith DA, Tweedie DJ, Vermeulen N, Williams DP, Wilson ID, Baillie TA. Managing the challenge of chemically reactive metabolites in drug development. Nat Rev Drug Discov. 2011;10(4):292–306. https://doi.org/10.1038/nrd3408.
Baillie TA, Pearson PG, Rashed MS, Howald WN. The use of mass spectrometry in the study of chemically-reactive drug metabolises. Application of MS/MS and LC/MS to the analysis of glutathione- and related S-linked conjugates of N-methylformamide. J Pharm Biomed Anal. 1989;7(12):1351–60. https://doi.org/10.1016/0731-7085(89)80140-2.
Jurva U, Wikström HV, Weidolf L, Bruins AP. Comparison between electrochemistry/mass spectrometry and cytochrome P450 catalyzed oxidation reactions. Rapid Commun Mass Spectrom. 2003;17(8):800–10. https://doi.org/10.1002/rcm.978.
Johansson T, Weidolf L, Jurva U. Mimicry of phase I drug metabolism – novel methods for metabolite characterization and synthesis. Rapid Commun Mass Spectrom. 2007;21(14):2323–31. https://doi.org/10.1002/rcm.3077.
Bussy U, Boujtita M. Advances in the electrochemical simulation of oxidation reactions mediated by cytochrome p450. Chem Res Toxicol. 2014;27(10):1652–68. https://doi.org/10.1021/tx5001943.
Bussy U, Boisseau R, Thobie-Gautier C, Boujtita M. Electrochemistry-mass spectrometry to study reactive drug metabolites and CYP450 simulations. TrAC Trends Anal Chem. 2015;70:67–73. https://doi.org/10.1016/j.trac.2015.02.017.
Krishnan S, Schenkman JB, Rusling JF. Bioelectronic delivery of electrons to cytochrome P450 enzymes. J Phys Chem B. 2011;115(26):8371–80. https://doi.org/10.1021/jp201235m.
Nouri-Nigjeh E, Permentier HP, Bischoff R, Bruins AP. Electrochemical oxidation by square-wave potential pulses in the imitation of oxidative drug metabolism. Anal Chem. 2011;83(14):5519–25. https://doi.org/10.1021/ac200897p.
Jahn S, Karst U. Electrochemistry coupled to (liquid chromatography/) mass spectrometry—current state and future perspectives. J Chromatogr A. 2012;1259:16–49. https://doi.org/10.1016/j.chroma.2012.05.066.
Jurva U, Weidolf L. Electrochemical generation of drug metabolites with applications in drug discovery and development. TrAC, Trends Anal Chem. 2015;70:92–9. https://doi.org/10.1016/j.trac.2015.04.010.
Bussy U, Giraudeau P, Silvestre V, Jaunet-Lahary T, Ferchaud-Roucher V, Krempf M, Akoka S, Tea I, Boujtita M. In situ NMR spectroelectrochemistry for the structure elucidation of unstable intermediate metabolites. Anal Bioanal Chem. 2013;405(17):5817–24. https://doi.org/10.1007/s00216-013-6977-z.
Boisseau R, Bussy U, Giraudeau P, Boujtita M. In situ ultrafast 2D NMR spectroelectrochemistry for real-time monitoring of redox reactions. Anal Chem. 2015;87(1):372–5. https://doi.org/10.1021/ac5041956.
Bussy U, Boujtita M. Review of advances in coupling electrochemistry and liquid state NMR. Talanta. 2015;136:155–60. https://doi.org/10.1016/j.talanta.2014.08.033.
Simon H, Melles D, Jacquoilleot S, Sanderson P, Zazzeroni R, Karst U. Combination of electrochemistry and nuclear magnetic resonance spectroscopy for metabolism studies. Anal Chem. 2012;84(20):8777–82. https://doi.org/10.1021/ac302152a.
Madsen KG, Olsen J, Skonberg C, Hansen SH, Jurva U. Development and evaluation of an electrochemical method for studying reactive phase-I metabolites: correlation to in vitro drug metabolism. Chem Res Toxicol. 2007;20(5):821–31. https://doi.org/10.1021/tx700029u.
Lohmann W, Karst U. Generation and identification of reactive metabolites by electrochemistry and immobilized enzymes coupled on-line to liquid chromatography/mass spectrometry. Anal Chem. 2007;79(17):6831–9. https://doi.org/10.1021/ac071100r.
Bussy U, Chung-Davidson YW, Li K, Li W. Phase I and phase II reductive metabolism simulation of nitro aromatic xenobiotics with electrochemistry coupled with high resolution mass spectrometry. Anal Bioanal Chem. 2014;406(28):7253–60. https://doi.org/10.1007/s00216-014-8171-3.
Bussy U, Chung-Davidson YW, Li K, Li W. A quantitative assay for reductive metabolism of a pesticide in fish using electrochemistry coupled with liquid chromatography tandem mass spectrometry. Environ Sci Technol. 2015;49(7):4450–7. https://doi.org/10.1021/es5057769.
Mali’n TJ, Weidolf L, Castagnoli Jr N, Jurva U. P450-catalyzed vs electrochemical oxidation of haloperidol studied by ultra-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2010;24(9):1231–40. https://doi.org/10.1002/rcm.4505.
Han J, Xian Z, Zhang Y, Liu J, Liang A. Systematic overview of aristolochic acids: nephrotoxicity, carcinogenicity, and underlying mechanisms. Front Pharmacol. 2019;10(648). https://doi.org/10.3389/fphar.2019.00648.
Vanherweghem JL, Tielemans C, Abramowicz D, Depierreux M, Vanhaelen-Fastre R, Vanhaelen M, Dratwa M, Richard C, Vandervelde D, Verbeelen D, Jadoul M. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. The Lancet. 1993;341(8842):387–91. https://doi.org/10.1016/0140-6736(93)92984-2.
Krumbiegel G, Hallensleben J, Mennicke WH, Rittmann N, Roth HJ. Studies on the metabolism of aristolochic acids I and II. Xenobiotica. 1987;17(8):981–91. https://doi.org/10.3109/00498258709044197.
Rendic SP, Guengerich FP. Human Family 1–4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: an update. Arch Toxicol. 2021;95(2):395–472. https://doi.org/10.1007/s00204-020-02971-4.
Chan W, Cui L, Xu G, Cai Z. Study of the phase I and phase II metabolism of nephrotoxin aristolochic acid by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20(11):1755–60. https://doi.org/10.1002/rcm.2513.
Pfau W, Schmeiser HH, Wiessler M. Aristolochic acid binds covalently to the exocyclic amino group of purine nucleotides in DNA. Carcinogenesis. 1990;11(2):313–9. https://doi.org/10.1093/carcin/11.2.313.
Stiborová M, Hudeček J, Frei E, Schmeiser H. Contribution of biotransformation enzymes to the development of renal injury and urothelial cancer caused by aristolochic acid: urgent questions, difficult answers. Interdiscip Toxicol. 2010;1(1):8–12. https://doi.org/10.2478/v10102-010-0023-1.
Clark WF, Garg AX. Plasma exchange for myeloma kidney: cast(s) away? Kidney Int. 2008;73(11):1211–3. https://doi.org/10.1038/ki.2008.117.
Wirtanen T, Rodrigo E, Waldvogel SR. Recent advances in the electrochemical reduction of substrates involving N−O bonds. Adv Synth Catal. 2020;362(11):2088–101. https://doi.org/10.1002/adsc.202000349.
Sidorenko VS, Attaluri S, Zaitseva I, Iden CR, Dickman KG, Johnson F, Grollman AP. Bioactivation of the human carcinogen aristolochic acid. Carcinogenesis. 2014;35(8):1814–22. https://doi.org/10.1093/carcin/bgu095.
Zanoni MVB, Rogers EI, Hardacre C, Compton RG. The electrochemical reduction of the purines guanine and adenine at platinum electrodes in several room temperature ionic liquids. Anal Chim Acta. 2010;659(1):115–21. https://doi.org/10.1016/j.aca.2009.11.026.
Takamura-Enya T, Suzuki H, Hisamatsu Y. Mutagenic activities and physicochemical properties of selected nitrobenzanthrones. Mutagenesis. 2006;21(6):399–404. https://doi.org/10.1093/mutage/gel045.
Author information
Authors and Affiliations
Contributions
UB conceptualized the research, contributed to the data interpretation, and drafted the manuscript. RB ran the experiments, contributed to the conceptualization of research, interpreted the data, and contributed to manuscript writing. MC contributed to experiment and data interpretation. RT contributed to manuscript writing. MB contributed to research conceptualization, supervised research, and contributed to writing.
Ethics declarations
Conflict of interest
UB is currently employed by Mars Incorporated. All the other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bussy, U., Boisseau, R., Croyal, M. et al. In-line formation and identification of toxic reductive metabolites of aristolochic acid using electrochemistry mass spectrometry coupling. Anal Bioanal Chem 414, 2363–2370 (2022). https://doi.org/10.1007/s00216-022-03874-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-03874-2