Abstract
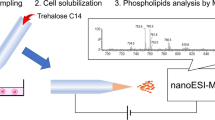
d-Allose, a rare, naturally occurring monosaccharide, is known to exert anti-proliferative effects on cancer cells. The effects of d-allose on the cellular membranes of hormone-refractory prostate cancer cell line (DU145), hormone-sensitive prostate cancer cell line (LNCaP), and normal prostate epithelial cells (PrEC) were studied at the molecular level by phospholipid (PL) profiling using a shotgun lipidomic method. The molecular structures of 85 PL species including 23 phosphatidylcholines, 12 phosphatidylethanolamines (PEs), 11 phosphatidylserines (PSs), 16 phosphatidylinositols, 9 phosphatidic acids (PAs), and 14 phosphatidylglycerols (PGs) were identified by data-dependent collision-induced dissociation of nanoflow liquid chromatography–tandem mass spectrometry, and the PL amounts were quantified. The addition of d-allose to prostate cancer cell lines during their growth phases had negligible or decreased effects on the relative regulation of PL species, but several new PS molecules (two for DU145 and three for LNCaP) emerged. In contrast, experiments on the PrEC cell line revealed that some high abundant species (14:0/14:0-PE, 16:2/16:0-PG, and 20:6/18:1-PA) showed significant increases in concentration. These findings support a mechanism for the anti-proliferative effect of d-allose on prostate cancer cell lines that involves the induction of programmed cell death since PS molecules are known to induce apoptosis. Principal component analysis was carried out to examine differences in PL distributions among the three cell lines promoted by d-allose.




Similar content being viewed by others
References
Brouwers JFHM, Vernooji EAAM, Tielens AGM, van Golde LMG (1999) J Lipid Res 40:164–169
Wright MM, Howe AG, Zaremberg V (2004) Biochem Cell Biol 82:18–26
Leach MO, Verrill M, Glaholm J, Smith TAD, Collins DJ, Payne GS, Sharp JC, Ronen SM, McCready VR, Powles TJ, Smith IE (1998) NMR Biomed 11:314–340
Bougnoux P, Chajes V, Lanson M, Hacene K, Body G, Couet C, Folch OL (1992) Breast Cancer Res Treat 20:185–194
Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, Elson P, Kennedy AW, Belinson J, Markman M, Casey G (1998) JAMA 280:719–723
Sutphen R, Xu Y, Wilbanks GD, Fiorica J, Grendys EC Jr, LaPolla JP, Arango H, Hoffman MS, Martino M, Wakeley K, Griffin D, Blanco RW, Cantor AB, Xiao YJ, Krischer JP (2004) Cancer Epidemiol Biomark Prev 13:1185–1191
Swanson MG, Vigneron DB, Tabatabai ZL, Males RG, Schmitt L, Carroll PR, James JK, Hurd RE, Kurhanewicz J (2003) Magn Reson Med 50:944–954
Min HK, Lim S, Chung BC, Moon MH (2011) Anal Bioanal Chem 399:823–830
Gronberg H (2003) Lancet 163:859–864
Clarke RA, Schirra HJ, Catto JW, Lavin MF, Gardiner RA (2010) Cancers 2:1125–1154
Hsing AW, Devesa SS (2001) Epidemiol Rev 23:3–13
Tamura K, Furihata M, Tsunoda T, Ashida S, Takata R, Obara W et al (2007) Cancer Res 67:5117–5125
Naha N, Lee HY, Jo MJ, Chung BC, Kim SH, Kim MO (2008) Apoptosis 13:1121–1134
Knudson CM, Korsmeyer SJ (1997) Nat Genet 16:358–363
Scher HI, Sawyers CL (2006) J Clin Oncol 23:8253–8261
Luck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) Science 275:1132–1136
Lee T, Malathi K, Lohyama S, Silane M, Berenstein A, Jayarama T (2004) Cell Biochem Funct 22:35–40
Arnold EC, Siladay PJ (1997) US Patent No. 5,620,960
Murata A, Sekiya K, Watanabe Y (2003) J Biosci Bioeng 96:89–91
Sui L, Dong Y, Watanabe Y et al (2005) Int J Oncol 27:907–912
Hirata Y, Saito M, Tsukamoto I, Yamaguchi F et al (2009) J Biosci Bioeng 107:562–568
Kim HY, Wang TCL, Ma YC (1994) Anal Chem 66:3977–3982
Koivusalo M, Haimi P, Heikinheimo L, Kostiainen R, Somerharaju P (2001) J Lipid Res 42:663–672
Fuchs B, Schiller J, Süβ R, Schürenburg M, Suckau D (2007) Anal Bioanal Chem 389:827–834
Taguchi R, Hayakawa J, Takeuchi Y, Ishida M (2000) J Mass Spectrom 35:953–966
Isaac G, Bylund D, Mansson J-E, Markides KE, Bergquist J (2003) J Neurosci Meth 128:111–119
Taguchi R, Houjou T, Nakanishi H, Yamazaki T, Ishida M, Imagawa M, Shimizu T (2005) J Chromatogr B 823:26–36
Bang DY, Kang D, Moon MH (2006) J Chromatogr A 1104:222–229
Bang DY, Ahn E, Moon MH (2007) J Chromatogr B 852:268–277
Ahn E, Kim H, Chung BC, Moon MH (2007) J Sep Sci 30:2598–2604
Kim H, Min HK, Kong G, Moon MH (2009) Anal Bioanal Chem 393:1649–1656
Min HK, Kong G, Moon MH (2010) Anal Bioanal Chem 396:1273–1280
Folch J, Less M, Stanley GHS (1957) J Biol Chem 226:497–509
Schroit AJ, Zwaal RF (1991) Biochim Biophys Acta 1071:313–3129
Iguchi K, Hirano K, Ishida RM (2010) Apoptosis 6:263–268
Chaurio RA, Janko C, Munoz LE, Frey B, Herrmann M, Gaipl US (2009) Molecules 14:4892–4914
Aussel C, Pelassy C, Breittmayer JP (1998) FEBS Lett 431:195–199
Uchida K, Emoto K, Daleke DL, Inoue K, Umeda M (1998) J Biochem 123:1073–1078
Acknowledgments
This study was supported by grant NRF-2010-0014046 and in part by grant NRF-2008-2003136 from the National Research Foundation of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, R.U., Lim, S., Kim, M.O. et al. Effect of d-allose on prostate cancer cell lines: phospholipid profiling by nanoflow liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 401, 689–698 (2011). https://doi.org/10.1007/s00216-011-5113-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5113-1