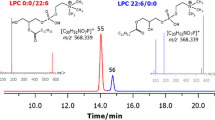
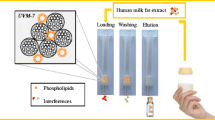
Abstract
A zirconia (ZrO2)-modified solid-phase extraction sorbent has been evaluated for selective extraction of phosphatidylcholines from biological samples, followed by analysis of the isolated solutes by reversed-phase liquid chromatography–electrospray ionization–tandem mass spectrometry. The clean-up process was optimized using seven standard phosphatidylcholines including two lyso derivatives. Different acidic conditions were tested for the bonding and washing steps; for elution, various aqueous or methanolic bases were studied. Experiments were conducted hydrodynamically using extraction cartridges, and statically in batch mode; the performance of the sorbent was significantly better when used in the flow-through mode. The developed clean-up procedure was used to selectively enrich phosphatidylcholines from whole milk, human plasma, and mouse plasma, to show the wide applicability of the method. For the preceding extraction of total lipids from the matrix, different solvent mixtures (methanol–chloroform, methanol–methyl tert-butyl ether, and ethanol–ethyl acetate) were compared. Accuracy and reproducibility of the proposed sample-preparation procedure were evaluated. Matrix effects possibly affecting mass spectrometric analysis were studied before and after the solid-phase extraction. They were found to be significant for several analytes, stressing the importance of a sample clean-up procedure. Under identical experimental conditions, recovery of bound phosphatidylcholines by zirconia was superior to that by other metal oxides, for example titania (TiO2) and stannia (SnO2).




Similar content being viewed by others
Abbreviations
- 16:0 lysoPC:
-
1-Palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine
- 18:0 lysoPC:
-
1-Stearoyl-2-hydroxy-sn-glycero-3-phosphocholine
- AcOH:
-
Acetic acid
- DAPC:
-
1,2-Diarachidonoyl-sn-glycero-3-phosphocholine
- DMPC:
-
1,2-Dimyristoyl-sn-glycero-3-phosphocholine
- DPPC:
-
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine
- ESI:
-
Electrospray ionization
- EtOAc:
-
Acetic acid ethyl ester
- EtOH:
-
Ethanol
- FA:
-
Formic acid
- GPL:
-
Glycerophospholipid
- HPLC:
-
High-performance liquid chromatography
- iPrOH:
-
2-Propanol
- LC:
-
Liquid chromatography
- MeOH:
-
Methanol
- MS:
-
Mass spectrometry
- MTBE:
-
Methyl tert-butyl ether
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PL:
-
Phosphoplipid
- PLPC:
-
1-Palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine
- POPC:
-
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- SPE:
-
Solid-phase extraction
- SRM:
-
Selected reaction monitoring
- TFA:
-
Trifluoroacetic acid
- QqQ:
-
Triple quadrupole
References
Peterson BL, Cummings BS (2006) Biomed Chromatogr 20:227–243
Pulfer M, Murphy RC (2003) Mass Spectrom Rev 22:332–364
Lessig J, Fuchs B (2009) Curr Med Chem 16:2021–2041
Schneider M (2001) Eur J Lipid Sci Technol 103:98–101
van Nieuwenhuyzen W, Tomas MC (2008) Eur J Lipid Sci Technol 110:472–486
Folch J, Lees M, Sloane SGH (1957) J Biol Chem 226:497–509
Bligh EG, Dyer WJ (1959) Can J Biochem Physiol 37:911–917
Perez-Palacios T, Ruiz J, Antequera T (2007) Food Chem 102:875–879
Siriamornpun S, Yang L, Kubola J, Li D (2008) J Food Lipids 15:164–175
Lopez C, Briard-Bion V, Menard O, Rousseau F, Pradel P, Besle J-M (2008) J Agric Food Chem 56:5226–5236
Parcerisa J, Codony R, Boatella J, Rafecas M (1999) J Agric Food Chem 47:1410–1415
Domingues MRM, Reis A, Domingues P (2008) Chem Phys Lipids 156:1–12
Larsen A, Hvattum E (2005) Mod Methods Lipid Anal Liq Chromatogr/Mass Spectrom Relat Tech 19–60
Schiller J, Suess R, Fuchs B, Mueller M, Zschoernig O, Arnold K (2006) Future Lipidol 1:115–125
Avalli A, Contarini G (2005) J Chromatogr A 1071:185–190
Chua SC, Tan CP, Lai OM, Long K, Mirhosseini H, Baharin BS (2008) Eur J Lipid Sci Technol 110:334–340
Han G, Ye M, Zou H (2008) Analyst 133:1128–1138
Blacken GR, Volny M, Diener M, Jackson KE, Ranjitkar P, Maly DJ, Turecek F (2009) J Am Soc Mass Spectrom 20:915–926
Kweon HK, Hkansson K (2006) Anal Chem 78:1743–1749
Yan J, Li X, Cheng S, Ke Y, Liang X (2009) Chem Commun 2929–2931
Qi D, Lu J, Deng C, Zhang X (2009) J Chromatogr A 1216:5533–5539
Ficarro SB, Parikh JR, Blank NC, Marto JA (2008) Anal Chem 80:4606–4613
Leitner A, Sturm M, Smatt J-H, Jaern M, Linden M, Mechtler K, Lindner W (2009) Anal Chim Acta 638:51–57
Li Y, Lin H, Deng C, Yang P, Zhang X (2008) Proteomics 8:238–249
Rivera JG, Choi YS, Vujcic S, Wood TD, Colon LA (2009) Analyst 134:31–33
Li Y, Qi D, Deng C, Yang P, Zhang X (2008) J Proteome Res 7:1767–1777
Pucci V, Di Palma S, Alfieri A, Bonelli F, Monteagudo E (2009) J Pharm Biomed Anal 50:867–871
Ikeguchi Y, Nakamura H (2000) Anal Sci 16:541–543
Calvano CD, Jensen ON, Zambonin CG (2009) Anal Bioanal Chem 394:1453–1461
Yang K, Zhao Z, Gross RW, Han X (2009) J Chromatogr B 877:2924–2936
Smatt J-H, Schuewer N, Jaern M, Lindner W, Linden M (2008) Micropor Mesopor Mater 112:308–318
Ardrey RE (2003) In: Ando DJ (ed) The effect of mobile phase additives and cone voltage. Wiley, Chichester, UK
Ardrey RE (2003) In: Ando DJ (ed) Structural information from electrospray ionization. Wiley, Chichester, UK
EURACHEM Guide (1998) A laboratory guide to method validation and related topics. LGC Ltd, Teddington, UK
Glisic SB, Skala DU (2009) Chem Ind Chem Eng Q 15:159–168
Ki I, Shibahara A, Yamamoto K, Nakayama T (1996) Lipids 31:535–539
Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jorgensen TJD (2005) Mol Cell Proteomics 4:873–886
Lin J-H, Liu L-Y, Yang M-H, Lee M-H (2004) J Agric Food Chem 52:4984–4986
Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D (2008) J Lipid Res 49:1137–1146
Mei H (2005) In: Korfmacher WA (ed) Matrix effects: causes and solutions. CRC Press, Boca Raton, USA
Jensen RG (2002) J Dairy Sci 85:295–350
Sanchez-Juanes F, Alonso JM, Zancada L, Hueso P (2009) Int Dairy J 19:273–278
Rombaut R, Camp JV, Dewettinck K (2005) J Dairy Sci 88:482–488
Rombaut R, Van Camp J, Dewettinck K (2006) Int J Food Sci Technol 41:435–443
Lilbaek HM, Fatum TM, Ipsen R, Sorensen NK (2007) J Agric Food Chem 55:2970–2978
Raffelt K, Moka D, Sullentrop F, Dietlein M, Hahn J, Schicha H (2000) NMR Biomed 13:8–13
Heimerl S, Liebisch G, Le Lay S, Boettcher A, Wiesner P, Lindtner S, Kurzchalia TV, Simons K, Schmitz G (2008) Biochem Biophys Res Commun 367:826–833
Li Z, Basterr MJ, Hailemariam TK, Hojjati MR, Lu S, Liu J, Liu R, Zhou H, Jiang X-C (2005) Biochim Biophys Acta/Mol Cell Biol Lipids 1735:130–134
Takatera A, Takeuchi A, Saiki K, Morisawa T, Yokoyama N, Matsuo M (2006) J Chromatogr B: Anal Technol Biomed Life Sci 838:31–36
Acknowledgment
A. Gonzálvez acknowledges financial support in the form of a F.P.U. grant (ref. AP-2007-04566) provided by the Ministerio de Ciencia e Innovación of Spain. The authors thank Dr Gerald Stübiger, Department of Vascular Biology and Thrombosis Research, Medical University of Vienna, for providing the plasma samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Dipl.-Ing. Dr Dr h.c. Professor Manfred Grasserbauer on the occasion of his 65th birthday
Rights and permissions
About this article
Cite this article
Gonzálvez, A., Preinerstorfer, B. & Lindner, W. Selective enrichment of phosphatidylcholines from food and biological matrices using metal oxides as solid-phase extraction materials prior to analysis by HPLC–ESI-MS/MS. Anal Bioanal Chem 396, 2965–2975 (2010). https://doi.org/10.1007/s00216-010-3527-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3527-9