Abstract
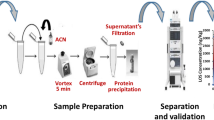
A rapid, sensitive, and specific method was developed and validated using liquid chromatography-tandem mass spectrometry for the simultaneous quantitation of atorvastatin (ATV) and its major metabolite ortho-hydroxyatorvastatin (o-HATV) in human plasma. The sample preparation involved a liquid–liquid extraction without chlorinated solvents and an on-line solid-phase extraction exploring the possibilities that anion exchange offers. The analytical method presented intraday and day-to-day variation below 10%; intraday and day-to-day accuracy stood between 94% and 105%; the limit of quantification was 0.1 ng/mL for ATV and 0.5 ng/mL for o-HATV; and the recovery was above 75% for both molecules. This method was applied successfully to quantitate ATV and o-HATV concentrations in an unstudied renal transplant recipient cohort treated with an immunosuppressive regime of tacrolimus and mycophenolic acid and a cohort of hypercholesterolemic patients included in the study as a control group. It can be used to evaluate patient adherence, drug–drug interactions, and pharmacokinetic/pharmacodynamic relationships. The results in our study showed that ATV and o-HATV levels in the renal transplant group were significantly increased (p < 0.001), compared to the hypercholesterolemic group.




Similar content being viewed by others
References
Bessler H, Salman H, Bergman M, Straussberg R, Djaldetti M (2005) Clin Immunol 117:73–77
Dunn SE, Youssef S, Goldstein MJ, Prod'homme T, Weber MS, Zamvil SS, Steinman L (2006) J Exp Med 203:401–412
Greenwood J, Mason JC (2007) Trends Immunol 28:88–98
Hermann M, Bogsrud MP, Molden E, Asberg A, Mohebi BU, Ose L, Retterstol K (2006) Clin Pharmacol Ther 79:532–539
Lea AP, McTavish D (1997) Drugs 53:828–847
Kantola T, Kivisto KT, Neuvonen PJ (1998) Clin Pharmacol Ther 64:58–65
Jemal M, Ouyang Z, Chen BC, Teitz D (1999) Rapid Commun Mass Spectrom 13:1003–1015
Bahrami G, Mohammadi B, Mirzaeei S, Kiani A (2005) J Chromatogr B 826:41–45
Zarghi A, Shafaati A, Foroutan SM, Khoddam A (2005) Arzneimittelforschung 55:451–454
Bullen WW, Miller PA, Hayes RN (1999) J Am Soc Mass Spectrom 10:55–66
Hermann M, Christensen H, Reubsaet JL (2005) Anal Bioanal Chem 382:1242–1249
Borek-Dohalsky V, Huclova J, Barrett B, Nemec B, Ulc I, Jelinek I (2006) Anal Bioanal Chem 386:275–285
Nirogi RV, Kandikere VN, Shukla M, Mudigonda K, Maurya S, Boosi R, Anjaneyulu Y (2006) Biomed Chromatogr 20:924–936
Lins RL, Matthys KE, Verpooten GA, Peeters PC, Dratwa M, Stolear JC, Lameire NH (2003) Nephrol Dial Transplant 18:967–976
Koal T, Deters M, Casetta B, Kaever V (2004) J Chromatogr B 805:215–222
Egge-Jacobsen W, Unger M, Niemann CU, Baluom M, Hirai S, Benet LZ, Christians U (2004) Ther Drug Monit 26:546–562
Buhrman DL, Price PI, Rudewicz PJ (1996) Am Soc Mass Spectrom 7:1099–1105
Salm P, Taylor PJ, Lynch SV, Warnholtz CR, Pillans PI (2005) Clin Biochem 38:667–673
Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, McKay G, Miller KJ, Patnaik RN, Powell ML, Tonelli A, Viswanathan CT, Yacobi A (2000) Pharm Res 17:1551–1557
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Anal Chem 75:3019–3030
Liu D, Jiang J, Zhou H, Hu P (2008) J Chromatogr Sci 46:862–866
Hermann M, Asberg A, Christensen H, Holdaas H, Hartmann A, Reubsaet JL (2004) Clin Pharmacol Ther 76:388–391
Wallemacq P, Armstrong VW, Brunet M, Haufroid V, Holt DW, Johnston A, Kuypers D, Meur YL, Marquet P, Oellerich M, Thervet E, Toenshoff B, Undre N, Weber LT, Westley IS, Mourad M (2009) Ther Drug Monit 31(2):139–152
Stern RH, Yang BB, Horton M (1997) J Clin Pharmacol 37:816–819
van Leuven SI, Kastelein JJ (2005) Expert Opin Pharmacother 6:1191–1203
Acknowledgments
We would like to thank Pfizer, Groton, USA for donating ATV, o-HATV and pHATV. CiberEHD and CiberOBN are funded by the Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guillén, D., Cofán, F., Ros, E. et al. Determination of atorvastatin and its metabolite ortho-hydroxyatorvastatin in human plasma by on-line anion-exchange solid-phase extraction and liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 394, 1687–1696 (2009). https://doi.org/10.1007/s00216-009-2852-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-2852-3