Abstract
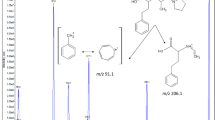
Cholesterol-reducing statin drugs are the most frequently prescribed agents for reducing morbidity and mortality related to coronary heart disease. In this publication a validated, highly sensitive, and selective isocratic HPLC method is reported for quantitative determination of the major statin drug atorvastatin (ATV) and its metabolite 2-hydroxyatorvastatin (HATV). Detection was performed with an electrospray ionization triple-quadrupole mass spectrometer equipped with an ESI interface operating in positive-ionization mode. Multiple reaction monitoring (MRM) was used for MS–MS detection. The calibration plot was linear in the concentration range 0.10–40.00 ng mL−1 for both ATV and HATV. Inter-day and intra-day precision and accuracy of the proposed method were characterized by measurement of relative standard deviation (RSD) and percentage deviation, respectively; both were less than 8% for both analytes. The limit of quantitation was 0.02 ng mL−1 for ATV and 0.07 ng mL−1 for HATV. The method was used for pharmacokinetic study of ATV and HATV. Pharmacokinetic data for all analytes are also reported.






Similar content being viewed by others
References
Lipitor Tablets, 10 mg, 20 mg, 40 mg, Summary of Product Characteristics, Parke–Davis, UK, October 2002
Sortis 10 mg, 20 mg, 40 mg, Souhrn údaj o přípravku (Summary of product characteristics in Czech), Parke– Davis, Germany, February 5, 2003
Lea AP, McTavish D (1997) Drugs 53:828–847
Kantola T, Kivistö KT, Neuvonen PJ (1998) Clin Pharmacol Ther 64:58–65
Lilja JJ, Kivistö KT, Neuvonen PJ (1999) Clin Pharmacol Ther 66:118–127
Ertuk S, Aktas ES, Ersoy L, Ficicioglu S (2003) J Pharm Biomed Anal 33:1017–1023
Erk N (2004) Crit Rev Anal Chem 34:1–7
van Pelt CK, Corso TN, Schltz GA, Lowes S, Henion J (2001) Anal Chem 73:582–588
Jemal M, Ouyang Z, Chen BC, Teitz D (1999) Rapid Commun Mass Spectrom 13:1003–1015
Bullen WW, Miller RA, Hayes RN (1999) J Am Soc Mass Spectrom 10:55–66
Posvar EL, Radulovic LL, Cilla DD, Whitfield LR, Sedman AJ (1996) J Clin Pharmacol 36:728–731
Radulovic LL, Cilla DD, Posvar EL, Sedman AJ, Whitfield LR (1995) J Clin Pharmacol 35:990–994
Atorvastatin Calcium, PDR for Lipitor Tablets, Pfizer Pharmaceuticals, November 2002. Available at: http://www.drugs.com/pdr/atorvastatin_calcium.html. Accessed: June 14, 2005
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bořek-Dohalský, V., Huclová, J., Barrett, B. et al. Validated HPLC–MS–MS method for simultaneous determination of atorvastatin and 2-hydroxyatorvastatin in human plasma—pharmacokinetic study. Anal Bioanal Chem 386, 275–285 (2006). https://doi.org/10.1007/s00216-006-0655-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0655-3