Abstract
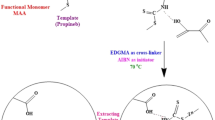
Molecularly imprinted polymers (MIPs) were prepared by precipitation polymerization using tebuconazole (TBZ) as a template. Frontal chromatography and selectivity experiments were used to determine the binding capabilities and binding specificities of different MIPs. The polymer that had the highest binding selectivity and capability was used as the solid-phase extraction (SPE) sorbent for the direct extraction of TBZ from different biological and environmental samples (cabbage, pannage, shrimp, orange juice and tap water). The extraction protocol was optimized and the optimum conditions were: conditioning with 5 mL methanol:acetic acid (9:1), 5 mL methanol and 5 mL water respectively, loading with 5 mL aqueous samples, washing with 1.2 mL acetonitrile (ACN):phosphate buffer (5:5, pH3), and eluting with 3 mL methanol. The MIPs were able to selectively recognize, effectively trap and preconcentrate TBZ over a concentration range of 0.5–15 μmol/L. The intraday and interday RSDs were less than 9.7% and 8.6%, respectively. The limit of quantification was 0.1 μmol/L. Under optimum conditions, the MISPE recoveries of spiked cabbage, pannage, shrimp, orange juice and tap water were 62.3%, 75.8%, 71.6%, 89% and 93.9%, respectively. MISPE gave better HPLC separation efficiencies and higher recoveries than C18 SPE and strong cation exchange (SCX) SPE.

HPLC analysis of spiked pannage after MISPE (A) and after C18 SPE (B). HQ (1), E3 (2), p-NP (3), FTF (4), TBZ (5), PNZ (6), HXZ (7)



Similar content being viewed by others
References
Stehmann C, Waard de MA (1996) Crop Prot 15:39–47
Sanagi MM, See HH, Ibrahim WAW, Naim AA (2004) J Chromatogr A 1059:95–101
US EPA (1999) Federal Register: Rules and Regulations 64:1132–1138
Paoli MD, Tacchedo M, Damiato V, Fabbro D Bruno R (1997) J Chromatogr A 765:127–131
Colume A, Cardenas S, Gallego M, Valcarcel M (2000) J Chromatogr A 882:193–203
Blasco C, Pico Y, Manes J, Font G (2002) J Chromatogr A 947:227–235
Blasco C, Pico Y, Font G (2002) J AOAC Int 3:704–710
Rodriguez R, Manes J, Pico Y (2003) Anal Chem 75:452–459
Zhu XL, Yang J, Su QD, Cai JB, Gao Y (2005) J Chromatogr A 1092:161–169
Osemwengie LI, Steinberg S (2001) J Chromatogr A 932:107–118
Xu F, Liang XM, Lin BC, Su F, Schramm KW, Kettrup A (1999) Chemosphere 39:2239–2248
Miyauchi T, Mori M, Ito K (2005) J Chromatogr A 1063:137–141
Redondo MJ, Ruiz MJ, Boluda R, Font G (1996) J Chromatogr A 719:69–76
Delaunay N, Pichon V, Hennion MC (2000) J Chromatogr B 745:15–37
Rashid BA, Aherne GW, Katmeh MF, Wasowski PK, Stevenson D (1998) J Chromatogr A 797:245–250
Hennion MC, Pichon V (2003) J Chromatogr A 1000:29–52
Sellergren B (1994) Anal Chem 66:1578–1582
Baggiani C, Trotta F, Giraudi G, Giovannoli C, Vanni A (1999) Anal Commun 36:263–269
Turiel E, Martin-Esteban A, Fernandez P, Perez-Conde C, Camara C (2001) Anal Chem 73:5133–5141
Mena ML, Martinez-Ruiz P, Reviejo AJ, Pingarron JM (2002) Anal Chim Acta 451:297–304
Matsui J, Okada M, Tsuruoka M, Takeuchi T (1997) Anal Commun 34:85–87
Zhu QZ, Degelmann P, Niessner R, Knopp D (2002) Environ Sci Technol 36:5411–5420
Ye L, Cormack PAG, Mosbac K (1999) Anal Commun 36:35–38
Jiang M, Zhang JH, Mei SR, Shi Y, Zou LJ, Zhu YX, Dai K, Lu B (2006) J Chromatogr A 1110:27–34
Theodoridis G, Zacharis CK, Tzanavaras PD, Themelis DG, Economou A (2004) J Chromatogr A 1030:69–76
Kasai K, Oda Y, Nishikata M, Ishii S (1986) J Chromatogr 376:33–42
Cheong SH, McNiven S, Rachkov A, Levi R, Yano K, Karube I (1997) Macromolecules 30:1317–1322
Tamayo FG, Casillas JL, Martin-Esteban A (2005) Anal Bioanal Chem 381:1234–1240
(1997) Method for the determination of triadimentol residues in cereals for export (SN0644)
Zhang JH, Jiang M, Zou LJ, Shi D, Mei SR, Zhu YX, Shi Y, Dai K, Lu B (2006) Anal Bioanal Chem 785:780–786
Wang J, Cormack PAG, Sherrington DC, Khoshdel E (2003) Angew Chem Int Ed 42:5336–5338
Tamayo FG, Casillas JL, Martin-Esteban A (2005) J Chromatogr A 1069:173–181
Olof R, Lars IA, Mosbach K (1993) J Org Chem 58:7562–7564
Barcelo D, Hennion MC (1995) Anal Chim Acta 318:1–41
Hennion MC, Coquart V (1993) J Chromatogr 642:211–219
Zander A, Findlay P, Renner T, Sellergren B (1998) Anal Chem 70:3304–3314
Theodoridis G, Manesiotis P (2002) J Chromatogr A 948:163–169
Andersson LI (2000) Analyst 125:1515–1517
Chapuis F, Pichon V, Lanza F, Sellergren B, Hennion MC (2003) J Chromatogr A 999:23–33
Karlsson JG, Andersson LI, Nicholls IA (2001) Anal Chim Acta 435:57–64
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 20307004 and NO. 20477013), and the Entry-Exit Inspection and Quarantine Bureau of PRC (NO 2005G0082).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material
Fig. S1
Scanning electron micrographs of the particles prepared: a the length of the bar is 1 μm; b the length of the bar is 5 μm (DOC 210 kb)
Rights and permissions
About this article
Cite this article
Hu, Ml., Jiang, M., Wang, P. et al. Selective solid-phase extraction of tebuconazole in biological and environmental samples using molecularly imprinted polymers. Anal Bioanal Chem 387, 1007–1016 (2007). https://doi.org/10.1007/s00216-006-1004-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-1004-2