Abstract
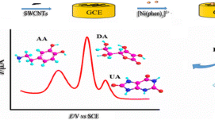
A simple, sensitive, and reliable method based on a combination of multi-walled carbon nanotubes with incorporated β-cyclodextrin (β-CD-MWNTs) and a polyaniline (PANI) film-modified glassy-carbon (GC) electrode has been successfully developed for determination of dopamine (DA) in the presence of ascorbic acid (AA). The PANI film had good anti-interference properties and long-term stability, because of the permselective and protective properties of the conducting redox polymer film. The acid-treated MWNTs with carboxylic acid functional groups promoted the electron-transfer reaction of DA and inhibited the voltammetric response of AA. Sensitive detection of DA was further improved by the preconcentration effect of formation of a supramolecular complex between β-CD and DA. The analytical response of the β-CD-MWNTs/PANI film to the electrochemical behavior of DA was, therefore, better than that of a MWNTs/PANI film, a PANI film, or a bare glassy-carbon (GC) electrode. Under the conditions chosen a linear calibration plot was obtained in the range 1.0 × 10−7–1.0 × 10−3 mol L−1 and the detection limit was 1.2 × 10−8 mol L−1. Interference from AA was effectively eliminated and the sensitivity, selectivity, stability, and reproducibility of the electrodes was excellent for determination of DA.







Similar content being viewed by others
References
Wightman R, Amatorh C, Engstrom R, Hale P, Kristensen E, Kubr W, May L (1988) Neuroscience 25:513–523
Wightman R, May L, Michael A (1988) Anal Chem 60:769A–770A
Adams R (1976) Anal Chem 48:1128A–1137A
Kawagoe K, Wightman R (1994) Talanta 41:865–874
Raj C, Okajima T, Ohsaka T (2003) J Electroanal Chem 543:127–133
Raj C, Tokuda K, Ohsaka T (2001) Bioelectrochemistry 53:183–191
Raj C, Ohsaka T (2001) J Electroanal Chem 496:44–49
Ciszewski A, Milczarek G (1999) Anal Chem 71:1055–1061
Mo J, Ogorevc B (2001) Anal Chem 73:1196–1202
Iijima S (1991) Nature (London) 354:56–58
Kuznetsova A, Mawhinney D, Naumenko V, Yates Jr J, Liu J, Smalley R (2000) Chem Phys Lett 321:292–296
Wang Z, Liu J, Liang Q, Wang Y, Luo G (2002) Analyst 127:653–658
Zhang P, Wu F, Zhao G, Wei X (2005) Bioelectrochemistry 67:109-114
Ly S (2006) Bioelectrochemistry 68:227–231
Zhang M, Gong K, Zhang H, Mao L (2005) Biosensors Bioelectron 20:1270–1276
Hoa D, Suresh Kumar T, Punekar N, Srinivasa R, Lal R, Contractor A (1992) Anal Chem 64:2645–2646
Sangodkar H, Sukeerthi S, Srinivasa R, Lal R, Contractor A (1996) Anal Chem 68:779–783
Qu F, Yang M, Jiang J, Shen G, Yu R (2005) Anal Biochem 344:108–114
Li G, Fang H, Chen H (1994) Chemical Research and Application, China, 6:7–12
Rekharsky M, Inoue Y (1998) Chem Rev 98:1875–1917
Zhang Q, Wang N, Zhan W, Xie F, Chen X (2003) Chinese Journal of Spectroscopy Laboratory 20:749–752
Wang Z, Wang Y, Luo G (2002) Analyst 127:1353–1358
Wang G, Liu X, Yu B, Luo G (2004) J Electroanal Chem 576:227–231
Wang Z, Xiao S, Chen Y (2006) J Electroanal Chem 589:237–242
He J, Yang Y, Yang X, Liu Y, Liu Z, Shen G, Yu R (2006) Sensors Actuators B 114:94–100
Bard A, Faulkner L (1980) (eds) Electrochemical methods. Wiley, New York
Murray R (1984) (eds) Electroanalytical chemistry. Marcel Dekker, New York, 13:191–368
Adams R (1969) J Pharm Sci 58:1171–1184
Malem F, Mandler D (1993) Anal Chem 65:37–41
Acknowledgements
This work was supported by Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, T., Wei, W. & Zeng, J. Selective detection of dopamine in the presence of ascorbic acid by use of glassy-carbon electrodes modified with both polyaniline film and multi-walled carbon nanotubes with incorporated β-cyclodextrin. Anal Bioanal Chem 386, 2087–2094 (2006). https://doi.org/10.1007/s00216-006-0845-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0845-z